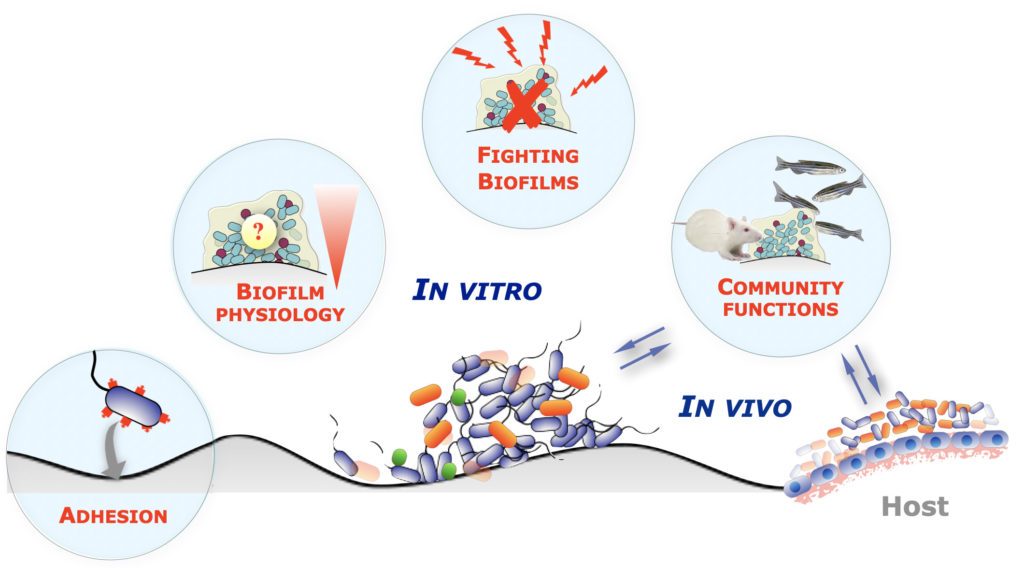
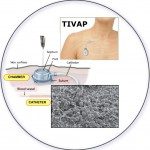
Biofilms are communities of microorganisms that interact with surfaces, and are known to display unique properties that differentiate them from free-floating, individual microorganisms. While biofilms are widespread and play many positive roles in all environments, they are also difficult to eradicate when adhered to surfaces in medical and industrial settings.
We use genetics, genomics, molecular biology, and various in vitro and in vivo biofilm models to better understand biofilm-associated functions used by commensal and pathogenic bacteria. We also attempt to translate our fundamental approaches into relevant anti-biofilm strategies in collaboration with clinicians and industrial partners.

Lopes AA, Vendrell-Fernandez S, Deschamps J, Georgeault S, Cokelaer T, Briandet R, Ghigo JM (2024) Bile-induced biofilm formation in Bacteroides thetaiotaomicron requires magnesium efflux by an RND pump. mBio,May 8;15(5):e0348823. doi: 10.1128/mbio.03488-23.
Thiriet-Rupert S, Josse J, Pérez-Pascual D, Tasse J, Andre C, Abad L, Lebeaux D, Ghigo JM, Laurent F, Beloin C (2023) Analysis of in-patient evolution of Escherichia coli reveals potential links to relapse of bone and joint infections. The Journal of Infectious Diseases, Dec 2:jiad528. doi: 10.1093/infdis/jiad528.
Grasekamp KP, Beaud B, Taib N, Audrain B, Bardiaux B, Rossez Y, Izadi-Pruneyre N, Lejeune M, Trivelli X, Chouit Z, Guerardel Y, Ghigo JM, Gribaldo S, Beloin C (2023) Bridges instead of boats? The Mla system of the diderm Firmicute Veillonella parvula is an ancestral envelope-spanning core of phospholipid trafficking. Nature Communications, Nov 23;.doi: 10.1038/s41467-023-43411-y.
Stevick RJ, Audrain B, Bedu S, Dray N, Ghigo JM, Pérez-Pascual D (2023) Anti-diarrheal drug loperamide induces dysbiosis in zebrafish microbiota via bacterial inhibition. Microbiome,Nov 11;11(1):252. doi:10.1186/s40168-023-01690-z.
Bernal-Bayard J, Thiebaud J, Brossaud MJ, Beaussart A, Caillet C, Waldvogel Y, Travier L,Létoffé S, Fontaine T, Rokbi B, Talaga P, Beloin C., Mistretta N*, Duval JFL*, Ghigo JM*. (2023) Bacterial capsular polysaccharides with antibiofilm activity share common biophysical and electrokinetic properties. Nature Communications. May 3;14(1):2553. doi: 10.1038/s41467-023-37925-8.
Masaru Usui, Yutaka Yoshii, Stanislas Thiriet-Rupert Jean-Marc Ghigo and Christophe Beloin. (2023) Intermitent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance. Communications Biology Mar 16;6(1):275. doi: 10.1038/s42003-023-04601-y.


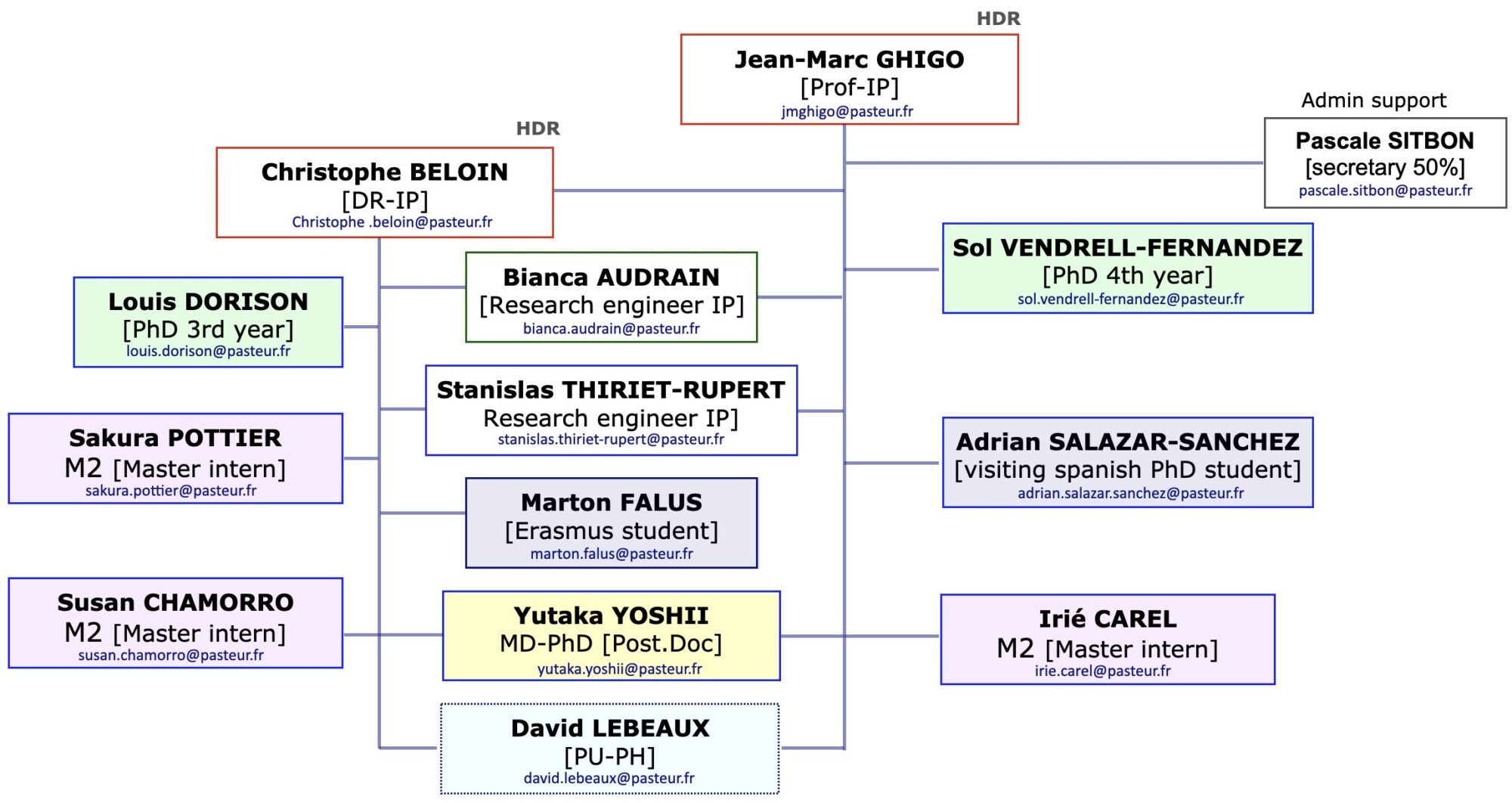
![]()