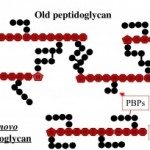
Our research unit aims at studying peptidoglycan (PGN) metabolism to better understand how bacteria assemble a mature and essential PGN that confers rigidity and shape to the cell despite a highly dynamic process to accompany cell growth and division.
We use Helicobacter pylori as a bacterial model since genome analysis indicates a minimal set of genes involved in PGN metabolism and assembly suggesting it might be a simpler model to study PGN metabolism. This seems to be confirmed by our recent work on the PGN hydrolases of H. pylori. The long-term goal of this part of my research is to better understand how bacteria coordinate PGN synthesis, and therefore to identify new therapeutic targets, but also to better understand the mechanisms of antibiotic resistance and their associated fitness cost. The study of fitness cost associated with antibiotic resistance is complementary to the discovery of new therapeutic targets as it can result in a better management of existing antibiotics and extend their shelf-live.
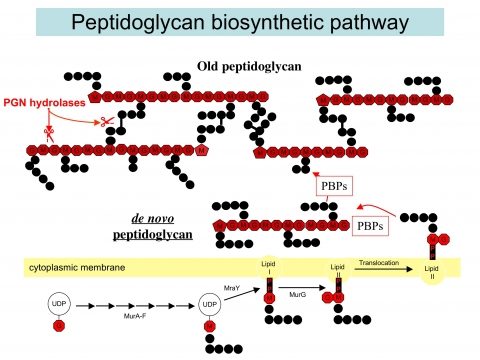
The second part of our research is aimed at studying the role of PGN in host-microbe interactions. Using several bacterial models, the objective is to understand how pathogens are able to subvert/modulate the host response by modifying their PGN, a major molecular pattern recognized by the host innate immune system. The different models include H. pylori, Neisseria meningitidis, Leptospira interrogans among others. For example, it is intriguing that an extracellular pathogen such as H. pylori acquired a virulence factor, a type-four secretion system, to deliver PGN fragments into host cells allowing its detection by the host. Hence, part of the project is aimed at studying the different bacterial virulence strategies exploring the biological activities of PGN. A second objective is to understand the dynamics of PGN sensing in the host cell during infection: which PGN structures are presented by the different pathogens, how the host detects them, responds to them and eventually detoxifies them. These studies are aimed at better understanding the role of PGN in pathogenesis, in determining what might differentiate a pathogen from a commensal organism, in the normal development of the gastrointestinal tract but also in the etiology of several chronic inflammatory conditions of the gastrointestinal tract (gastric cancer or Crohn’s disease). Fianly, we study the beneficial effects of commensal or probiotic bacteria on the host physiology in particular during a dysbiosis associated with cancer and anticancer therapy.