The nicotinic receptor is at the basis of nicotine addiction, which presents a serious social and public health problem. It is the single most important preventable factor of mortality and morbidity worldwide. More than 100 million people are expected to die this century from the consequences of smoking, and also second hand smoke.
But it is also a major player in a number of other pathologies, including Alzheimer’s disease, Parkinson’s disease, schizophrenia, multiple sclerosis and ALS. Hence, the identification of the molecular mechanisms and circuits involved urgently requires the development of novel tools allowing genetic and molecular manipulation in vivo, in experimental animals, and human induced pluripotent stem cells (hiPSC).
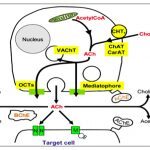
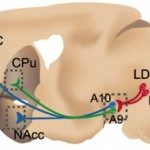
Over the last years, we have based most of our work on robust Genome-wide Association Studies (GWAS) linking human polymorphisms in genes coding for the nicotinic acetylcholine receptor (nAChR) to smoking. We have focused on a coding Single Nucleotide Polymorphism (SNP) in the CHRNA5 gene, coding for the alpha5 nAChR subunit, and dissected its role in reinforcement, consumption levels, and relapse. Analysing the role of a second gene linked through GWAS, CHRNB4, coding for the beta4 nAChR, we were able to identify a new circuit determining nicotine intake, and implicating the medial habenula-interpeduncular pathway. These findings have led to an approach of “precision medicine”.
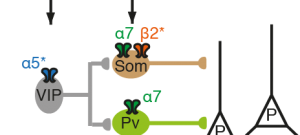
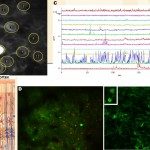
A second pathology addressed is schizophrenia, and its relationship with heavy smoking. There also, large-scale GWAS identified the same human polymorphisms, a haplotype on chromosome 15q. Using advanced two-photon imaging in the wake behaving mouse, we were able to identify a network of cortical interneurons expressing nAChRs, and a key role for the alpha5 SNP in reduced cortical activity reminiscent of “hypofrontality” in human patients. This altered activity is restored by the application of chronic nicotine, lending support to the “self-medication” hypothesis, i.e. psychiatric patients smoke to alleviate symptoms of the disease.
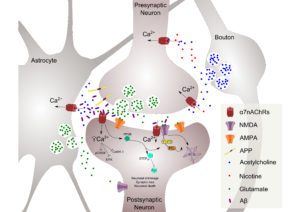
We have also been able to elucidate a role for nAChRs in Alzheimer’s disease (AD), where a “protective” role of nicotine, or even smoking, is debated. In animal models, AD-like pathology is reduced in the absence of the high affinity beta2 nAChR subunit. This is the subunit responsible for the high affinity binding of nicotine, and is “desensitised” in the brains of smokers. This has led to a patent, and a novel strategy to prevent the progression of the disease.
Finally, GWAS and meta-analysis have also revealed a role for the same alpha5 nAChR SNP in lung cancer and Chronic Obstructive Pulmonary Disease (COPD), one of the main unresolved diseases worldwide. This work, implicating “neuronal” nAChRs in non-neuronal, bronchial epithelial stem cells, is supported by major French gouvernmental programmes (Plan Cancer).
Highlights for future research include:
⎫ Comprehensive mechanistic analysis in several transgenic rat models
⎫ A novel system for the study of human iPSC derived neurons in vivo
⎫ An approach for personalised medicine targeting human nAChR polymorphisms
⎫ A novel approach to target Alzheimer’s disease
⎫ Biomedical research on the role of the nicotinic receptor in lung cancer and COPD
⎫ The nicotinic receptor and its relationship with the microbiome and immune system