Vector-borne diseases are among the leading causes of morbidity and mortality for humans and animals; An arthropod vector is needed for their transmission. For example, malaria caused by a protozoan transmitted by mosquitoes of the Anopheles genus, is responsible of more than 600,000 deaths per year, and dengue fever caused by a virus transmitted by mosquitoes of the Aedes genus affects 390 million people every year. Emergence of these vector-borne diseases depends on the evolution of interactions between mosquitoes and microbes they transmit. Disruption of ecosystems, changes in demography, intensification of international trade, climate change are among the factors influencing these interactions.
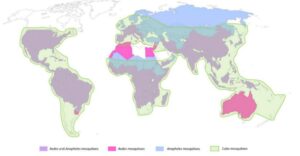
Distribution of mosquitoes of the genera Aedes, Anopheles and Culex (Viglietta et al. Frontiers in Microbiology, 2021)
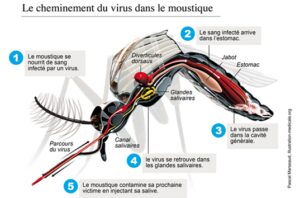
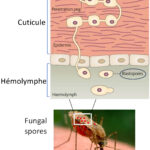
The mosquito becomes infected when taking a blood meal from a vertebrate host. The pathogen (parasite, virus, etc.) present in the food bolus migrates from the mosquito’s stomach to the salivary glands by successfully crossing the physical barriers (stomach and salivary glands) and the molecular barriers (immune system) of the mosquito. Once in saliva, the virus or the parasite is transmitted to the host by bite. Once in the salivary glands of the mosquito, the microbial agent remains there throughout the life of the vector and can thus be transmitted to a vertebrate host with each new bite.
Development of a pathogen in a vector: example of the virus in the mosquito
Arboviruses (viruses transmitted by arthropods) are responsible for zoonoses in very complex forest and rural ecosystems involving, in sylvatic cycles, numerous vector species and a wide variety of animals. Humans become accidentally contaminated during repeated contact with wildlife and zoophilic mosquitoes (usually biting animals) found there. Epidemics occur when the virus is introduced into an urban environment where anthropophilic vectors (biting humans) and a large susceptible human population coexist. There are more than 500 arboviruses, around which a hundred are pathogenic for humans. From a taxonomic point of view, arboviruses form a heterogeneous group of viruses belonging to different viral families. Arboviruses come mainly from seven viral families: Flaviviridae, Togaviridae, Phenuiviridae, Peribunyaviridae, Nairoviridae, Rhabdoviridae and Reoviridae. These viruses differ in genome type, structural organization and replication strategy. With the exception of African swine fever virus (Family Asfarviridae, Genus Asfivirus) which is a DNA virus, arboviruses have an RNA molecule as genetic material. Depending on the characteristics of the viral family, this RNA can be segmented or not, single or double stranded and in the case of single strands, of positive or negative polarity.
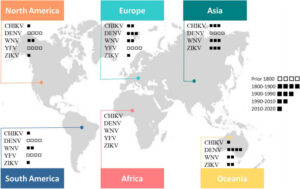
CHIKV (chikungunya) : Alphavirus, Togaviridae ; DENV (dengue) : Flavivirus, Flaviviridae ; WNV (West Nile): Flavivirus, Flaviviridae ; YFV (fièvre jaune) : Flavivirus, Flaviviridae ; ZIKV (Zika) : Flavivirus, Flaviviridae.
World distribution of the most important arboviruses for human health (Bellone et al., Frontiers in Microbiology, 2020)
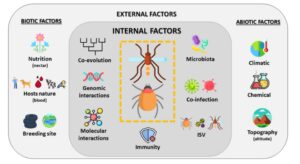
Main factors affecting the vectorial system, the virus, the mosquito and the vertebrate (human or animal) (Viglietta et al. Frontiers in Microbiology, 2021)
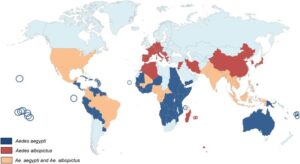
Distribution of anthropophilic mosquitoes Aedes aegypti and Aedes albopictus in 2019 (Houé et al. Emerging Microbes & Infections, 2019)
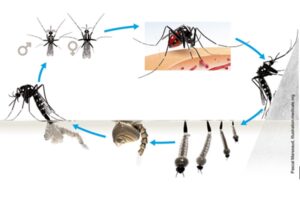
Mosquito development cycle
In this context, our AIV unit collaborates in a privileged way with the institutes of the Pasteur Network which today has 33 institutes spread over the 5 continents. Our work aims to understand the factors leading to the emergence of pathogens by focusing on the interactions between the pathogen, the mosquito vector and the environment. More precisely, our objective is to analyze the combined effects of genetic (immune system) and non-genetic (environmental) factors influencing the vector competence of Aedes and Anopheles mosquitoes against respectively arboviruses and the parasite responsible for malaria, Plasmodium. We have insectaries to raise mosquitoes Aedes (aegypti, albopictus, vexans), Culex (pipiens pipiens, pipiens molestus, quinquefasciatus) Anopheles (atroparvus, coluzzii, funestus), an infrastructure to carry out experimental infections in BSL-3, an A3 animal facility to carry out transmission tests from the infected mosquito to the animal, and facilities to handle MOT (micro-organisms and toxins).
Furthermore, current vector control strategies are strongly hampered by the development and increased prevalence of mosquito resistance to insecticides. Since their early stages of development, mosquitoes are subject to rich and varied microbial pressure. Using our knowledge of the antimicrobial immune response of mosquitoes, our unit is working on the development of translational approaches with the objective, not of reducing mosquito populations, but rather of reducing their vector competence.