From 2001 to 2005 I did my Ph.D in Montpellier (South of France) at the Institut of Functional Genomic. I worked on Drosophila melanogaster and I characterized a new G-Protein Coupled Receptor, only found in insects. I found both the ligand and the function of this orphan receptor we called “mange tout“.
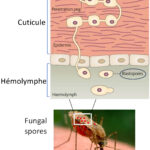
In October 2005, I joined the Institut Pasteur for my first Postdoctoral training for working on another insect diptera, the mosquito species, Anopheles gambiae. This mosquito species is the main vector of the malaria parasite in Africa, where Plasmodium falciparum that is mainly found. Malaria is still responsible for the death of more than 600,000 people each year. From 2005 to 2008, I settled an automated culture system for producing P. falciparum gametocytes and initiated functional genomic studies to identify mosquito genes controlling the development of P. falciparum during its sporogonic cycle. In 2009, I started a second postdoc in the unit of Dr. Kenneth D. Vernick to work on Anopheles immunity. In 2011, I got a permanent position as a researcher at the Institut Pasteur and developed different project related mosquito exposures and immunity to distinct microbes, and their influence on Anopheles vector competence for the malaria parasite Plasmodium. This cross-influence of microbial exposure on subsequent infection in insect is know as immune priming. For the microbial exposure, we used the entomopathogenic fungi (kill insects) and the Trypanosoma parasites, which are both present in area where Malaria is endemic.
In February 2024, I joined the team of Anna-Bella Failloux as a group leader. I will pursue the project related to mosquito immune priming in Anopheles, but will also open my field of research interest to Aedes mosquitoes, which are vectors of arboviruses, but does not transmit the human malaria parasites. The genetic and genomic determinants as well as the mechanisms of the vectorial specificity are poorly understood. Identifying the molecular factors as well as the mechanisms underlying mosquito immunity and their vectorial specificity could help designing novel strategies to control microbial transmission mediated by distinct mosquito vector species.