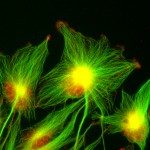
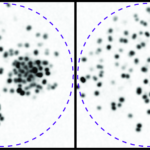
Our research focuses on cell polarization and migration in health and disease and more specifically on the regulatory mechanisms of astrocyte migration in the context of inflammation and glioblastoma invasion. Astrocytes are major glial cells of the central nervous system. They fulfil a wide variety of functions allowing neurons to develop, survive and function correctly. In a normal adult brain, astrocytes are essentially immobile and do not display any obvious polarized morphology. Under pathological situations involving inflammation of the cerebral tissue, astrocytes become reactive and polarize and migrate in a collective manner in the direction of the inflammatory site. In these conditions, cell polarization and migration are tightly regulated by the extracellular environment. Astrocytes or their precursors can give rise to very invasive tumors called gliomas. Gliomas correspond to the majority of primary brain tumors and are associated with very poor prognosis. The most aggressive gliomas, called glioblastomas, are amongst the most invasive tumors. The capacity glioblastoma cells to escape the initial tumor and migrate over long distance allow them to escape to classical therapeutic treatments.
Our aims are to identify the extracellular cues that act on astrocytes to control the speed and direction of migration, to decipher the fundamental polarity signaling which controls cell polarization during migration, and to determine how signalling cascade influence the organization of cytoskeletal elements to eventually promote cell migration.
In vitro and in vivo models are used to compare the directed collective migration of normal astrocytes to the invasion of glioblastoma cells in order to identify molecular alterations responsible for the loss of polarity and abnormal migratory behaviour of glioblastoma cells.