Présentation
CyaA, a 1706 residue-lo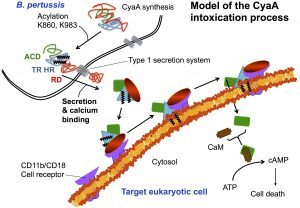
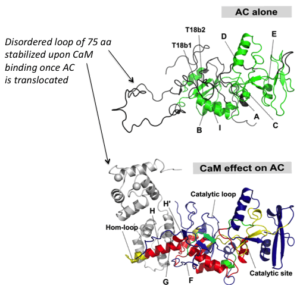
One challenging aspect of the structural and biophysical studies of CyaA arises from the complexity of this toxin, a large (1706 amino-acids) multi-domain protein that is post-translationally acylated and exhibits a pronounced hydrophobic character limiting its solubility. Lately, our work has focused on the characterization of individual domains of the toxin, mainly the N-terminal catalytic domain (AC) and the C-terminal Repeat-in-ToXin (RTX) Domain (RD and part of it) using a combination of biochemical and biophysical approaches (more details on the BIM site and on the group site).
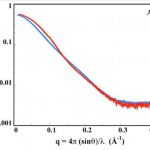
We have recently described a procedure to produce a monomeric, stable, soluble and functional state of the full-length CyaA toxin. We are now investigating the physico-chemical properties of CyaA in solution and upon its insertion into membranes.
The characterization of CyaA in solution should be instrumental for the development of a new generation of vacines against whooping cough. Biophysical approaches will be coined to follow the translocation process both in vitro on lipid membranes and in vivo on eukaryotic cells. These studies should provide a better understanding of the mechanisms of toxin translocation across biological membranes, and in addition, will allow to further develop CyaA-based vaccines (two of them are currently in phase I/II clinical trials). Indeed, Daniel Ladant, in collaboration with C. Leclerc’s team at Institut Pasteur, previously showed that CyaA is a potent vehicle able to deliver vaccine antigens into dendritic cells thus triggering specific cell-mediated immune responses (more details here).
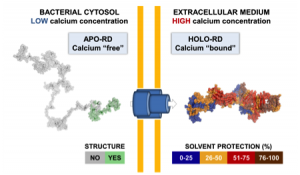
From a basic point of view, our project will provide new insights into the behavior of the toxin both in solution and upon interaction with membranes. We will be able to solve several key unanswered questions: (i) how the acylation of CyaA and calcium-binding can affect the protein conformation and stability; (ii) how these changes can favor the association of CyaA with the membrane and the translocation of its catalytic domain across the lipid bilayer; (iii) how the nature of the lipids (charge, acyl chain length, lipid polymorphism…) and physico-chemical parameters (ionic strength, membrane potential…) may modulate the successive steps leading to the translocation of the catalytic domain; (iv) how the presence of the CD11b/CD18 receptor may modulate the translocation process.
From an applied perspective, a better understanding of the physico-chemical properties of CyaA will contribute to improve all biotechnological aspects pertaining to the manufacturing of CyaA-based therapeutic molecules developed in the BIM Unit. This includes improvement of production processes, quality-control procedures and formulation of the recombinant proteins for vaccines and clinical uses. Finally, an in-depth description of the translocation process will provide new opportunities to improve the efficiency and safety of CyaA as an antigen delivery vehicle.