Life is maintained through alternating phases of cell division and quiescence. The causes and consequences of spontaneous mutations have been extensively explored in proliferating cells, and the major sources include errors of DNA replication and DNA repair. The foremost consequences are genetic variations within a cell population that can lead to heritable diseases and drive evolution. While most of our knowledge on DNA damage response and repair has been gained through cells actively dividing, it remains essential to also understand how DNA damage is metabolized in cells which are not dividing.
Age-related diseases including cancer and neurodegeneration are the major threats to human health in developed countries and represent a challenge for individual patient care and public health systems. Somatic mutations cause not only cancer but also abnormalities in the brain and recent findings indicate that a range of age-related diseases are linked to defective DNA repair pathways (Garinis et al. 2008; Burtner and Kennedy 2010). Genetic instability has been implicated in several neurodegenerative disorders, including amyotrophic lateral sclerosis (Julien 2001; Lim et al. 2006), and it has been proposed that the highly metabolically active neurons are particularly sensitive to DNA damage and that their post-mitotic lifespan relies on specific DNA surveillance and repair systems. Therefore, the stability and homeostasis of the genetic material in post-mitotic tissues may play a greater role than anticipated, and raises the question of how DNA damage is repaired in non-dividing cells.
All organisms exist in two cell states: proliferation, whereby the cell number increases by division, and quiescence, which sustains life during the non-dividing phase. The majority of the cells in human body tissues and organs are non-dividing, so quiescence is a common life form for the cell. However, this latter state contains two separate conditions depending upon their capacity to re-enter the cell cycle. For example, the yeast model systems and stem cells sustain a reversible quiescent state and can resume proliferation, whereas post-mitotic cells are metabolically active, but irreversibly arrested.
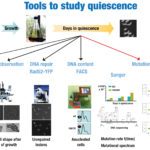
Interestingly, several recent developments, including the ability to use genome-scale tools to understand this state, have facilitated studies into the quiescent state, especially in yeast. To understand these important biological functions better, the mechanisms of quiescence and particularly those pertaining to the repair capabilities of the quiescent cells must be studied. Indeed, cells must have evolved strategies to protect and neutralize physical and chemical attacks as well as DNA repair mechanisms to prevent the accumulation of DNA damage responsible for mutations and cell death, allowing them to survive through division or arrest.