Biophysical investigations of the Bordetella pertussis adenyl cyclase (CyaA) toxin. This project is performed by Dorothée Raoux Barbot, Gaia Scilironi, Mirko Sadi, Minh-Ha Nguyen, Amiel Abettan, Corentin Leger, Nicolas Carvalho, Maryline Davi, Daniel Ladant and Alexandre Chenal. Past members of the group are Alexis Voegele, Darragh O’Brien, Mélanie Huet, Ana Cristina Sotomayor Pérez, Johanna C. Karst, Orso Subrini, Anna Wozniak, Audrey Hessel, Sylvain Debard, Sara Elisabetta Cannella and Véronique Yvette Ntsogo. Colleagues form other groups involved in the project are listed here.
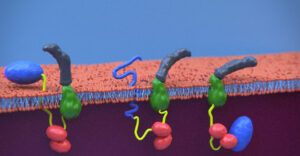
Scheme of CyaA translocation across the plasma membrane (adapted from Advance Sciences 2021)
Our research interests are mainly focused on the study of the molecular mechanisms that underlying protein folding and membrane translocation of a bacterial toxin, the adenylate cyclase (CyaA) produced by Bordetella pertussis, the causative agent of whooping cough, which is currently in increasing incidence and represents a global public health concern. The study of CyaA offers the opportunity to explore various topics such as intrinsically disordered proteins (IDP), molecular crowding, protein-protein, protein-ligand and protein-membrane interactions.
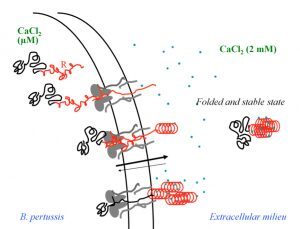
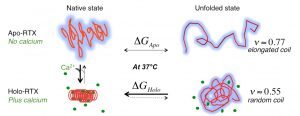
Secretion and calcium-induced folding of the RTX domain of CyaA
CyaA, a 1706 residue-long protein, is one of the major virulence factors produced by B. pertussis and plays an important role in the early stages of respiratory tract colonization. This toxin uses an original intoxication mechanism: secreted by the virulent bacteria, CyaA is able to invade eukaryotic target cells through a unique but poorly understood mechanism that involves a calcium-dependent direct translocation of its N-terminal catalytic domain across the plasma membrane. Then, upon activation by the endogenous cytosolic calmodulin (CaM), CyaA catalyzes massive production of cAMP that in turn alters cellular physiology. Our main objective is to unravel the molecular mechanisms of this unique entry pathway.
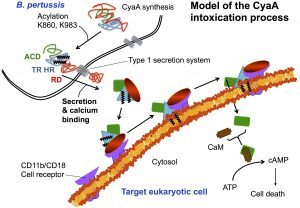
One challenging aspect of the structural and biophysical studies of CyaA arises from the complexity of this toxin, a large (1706 amino-acids) multi-domain protein that is post-translationally acylated and exhibits a pronounced hydrophobic character limiting its solubility. Until recently, the structural data available on the protein was the 3D structure of the catalytic domain in complex with the C-terminal part of calmodulin solved by the group of Wei-Jen Tang. In the last times, our work has been focused on the characterization of individual domains of the toxin. We have characterized the N-terminal catalytic domain, alone and in complex with full-length calmodulin (see below). In particular, we show that a loop of more than 70 amino-acids is unfolded in AC, favoring both secretion and translocation processes. This structural disorder is further used as a bait to capture calmodulin in the eucaryotic cytosol. Calmodulin binding induces folding of the H-helix of AC and a strong stabilization of the AC:CaM complex, leading to the enzymatic activation of AC (approx. 2000 ATP converted to cAMP per second while in the resting state, AC kcat is 1 cAMP/sec). 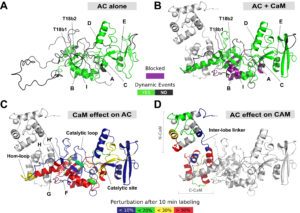
Calmodulin-induced disorder to order transition in AC leads to its enzymatic activation.
We also investigated the C-terminal Repeat-in-ToXin (RTX) Domain (calcium-induced disorder to order transition in RD, see above) using a combination of biochemical and biophysical approaches (more details here). We showed that the negatively charged RTX domain is characterized by an elongated conformation, with dimensions appropriated for its uptake and secretion through the narrow channel of the Type 1 secretion system. Once the C-terminal part of RD reaches the extracellular medium, calcium binding induces the folding of RD into a stable domain. We further showed that once RD is refolded, it acts as a scaffold for the refolding of the N-terminal domains that are progressively secreted from the T1SS. 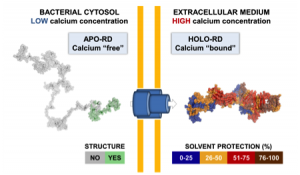
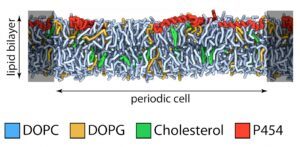
We also study the. translocation process, in particular, the early stages of the translocation process, which involves the “translocation domain” of CyaA (approx. residues 370-500). This region contains several amphitropic segments and some of them, with properties related to antimicrobial peptides. We hypothesize that such segment is involved in local membrane perturbation, decreasing the energy required to translocate AC across the plasma membrane.
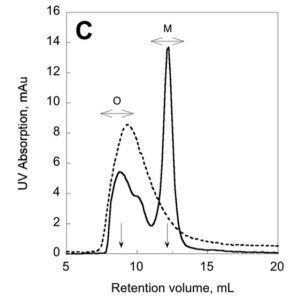
We have recently described a procedure to produce a monomeric, stable, soluble and functional state of the full-length CyaA toxin. We are now investigating the physico-chemical properties of CyaA in solution and upon its insertion into membranes.
Production of a monomeric, stable and functional holo-CyaA species (M)
The characterization of CyaA in solution should be instrumental to develop a new generation of vacines against whooping cough. Biophysical techniques will be developed to follow the translocation process both in vitro on lipid membranes and in vivo on eukaryotic cells. These studies should provide a better understanding of the mechanisms of toxin translocation across biological membranes, and in addition, will be instrumental for further developments of CyaA-based vaccines (two of them are currently in phase I/II clinical trials). Indeed, Daniel Ladant, in collaboration with C. Leclerc’s team at Institut Pasteur, previously showed that CyaA is a potent vaccine vehicle able to deliver antigens into dendritic cells to trigger specific cell-mediated immune responses (more details here). Besides investigating the biophysics of CyaA, I have pursued several projects initiated during my previous post-doctoral positions or within new collaborations established with various groups inside or outside Institut Pasteur (more details here).