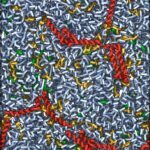
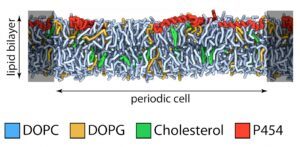
Biophysical investigations of the Bordetella pertussis adenyl cyclase (CyaA) toxin. This project is performed by Dorothée Raoux Barbot, Alexis Voegele, Mélanie Huet, Mirko Sadi, Darragh O’Brien, Maryline Davi, Daniel Ladant and Alexandre Chenal. Past members of the group are Ana Cristina Sotomayor Pérez, Johanna C. Karst, Orso Subrini, Anna Wozniak, Audrey Hessel, Sylvain Debard, Sara Elisabetta Cannella and Véronique Yvette Ntsogo. Colleagues form other groups involved in the project are listed here. Our research interests are mainly focused on the study of the molecular mechanisms that underlying protein folding and membrane translocation of a bacterial toxin, the adenylate cyclase (CyaA) produced by Bordetella pertussis, the causative agent of whooping cough, which is currently in increasing incidence and represents a global public health concern. The study of CyaA offers the opportunity to explore various topics such as intrinsically disordered proteins (IDP), molecular crowding, protein-protein, protein-ligand and protein-membrane interactions.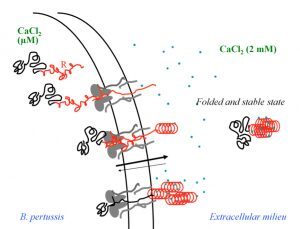
Cliquez pour voir le graph
Connexions
Membres

Alexandre Chenal
Chef(fe) de Groupe
Responsable
Anciens Membres
2000
2000
Name
Position
2015
2020
Orso Subrini
Post-doc
2015
2020
Audrey Hessel
Post-doc
2015
2020
Ana Cristina Sotomayor Pérez
Post-doc
2015
2020
Johanna Karst
Post-doc
2015
2020
Véronique Yvette Ntsogo Enguéné
PhD Student
2015
2020
Sara Elisabetta Cannella
PhD Student
2015
2020
Darragh O’Brien
Post-doc
2015
2019
Alexis Voegele
PhD Student
2016
2018
Mélanie Huet
BTS Tech
Projets
Projet Transversal
Financements
Publications
Télécharger-
2020Development of Conformational Antibodies to Detect Bcl-xL’s Amyloid Aggregates in Metal-Induced Apoptotic Neuroblastoma Cells., Int J Mol Sci 2020 Oct; 21(20): .
-
2020Dissecting the Structural and Chemical Determinants of the “Open-to-Closed” Motion in the Mannosyltransferase PimA from Mycobacteria., Biochemistry 2020 Aug; 59(32): 2934-2945.
-
2020Essential dynamic interdependence of FtsZ and SepF for Z-ring and septum formation in Corynebacterium glutamicum., Nat Commun 2020 Apr; 11(1): 1641.
-
2020Hydrogen/Deuterium Exchange Mass Spectrometry for the Structural Analysis of Detergent-Solubilized Membrane Proteins., Methods Mol Biol 2020 ; 2127(): 339-358.
-
2020Functional and structural consequences of epithelial cell invasion by Bordetella pertussis adenylate cyclase toxin., PLoS ONE 2020 ; 15(5): e0228606.
-
2019Post-translational acylation controls the folding and functions of the CyaA RTX toxin., FASEB J 2019 09; 33(9): 10065-10076.
-
2019The Adenylate Cyclase (CyaA) Toxin from Bordetella pertussis Has No Detectable Phospholipase A (PLA) Activity In Vitro., Toxins (Basel) 2019 02; 11(2): .
-
2018Translocation and calmodulin-activation of the adenylate cyclase toxin (CyaA) of Bordetella pertussis., Pathog Dis 2018 Nov; 76(8): .
-
2017Structural disorder and induced folding within two cereal, ABA stress and ripening (ASR) proteins, Sci Rep 2017 Nov;7(1):15544.
-
2017Membrane-Active Properties of an Amphitropic Peptide from the CyaA Toxin Translocation Region, Toxins (Basel) 2017 Nov;9(11).
-
+Voir la liste complète de publications
Contact
Address
25-28 Rue du Docteur Roux 75015,
Paris France