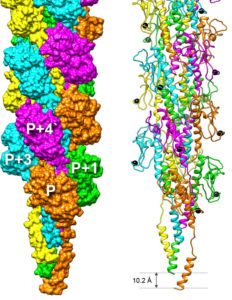
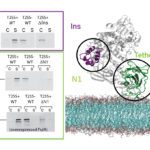
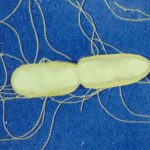
The Macromolecular systems team seeks to understand the molecular mechanisms of protein transport and organelle biogenesis in Gram-negative bacteria. We use mainly Escherichia coli and Klebsiella as model organisms. Our current focus is on the basic mechanisms of folded protein transport by the Type II secretion system and on the assembly of surface filaments called Type IV pili. Both of these complex nanomachines share similar protein components and assemble flexible filaments from protein subunits initially embedded in the inner membrane. In the Type II secretion system, these filaments (called endopili) promote secretion of fully folded proteins from the periplasm across the bacterial outer membrane. Type IV pili in Enterohaemorrhagic Escherichia coli (EHEC) are implicated in bacterial adhesion to surfaces, and are co-regulated with DNA uptake and natural competence machinery. More recently we have been involved in a collaborative projects funded by the ANR-NSF and HFSP to understand the mechanisms of nanowire secretion and assembly in soil bacteria such as Geobacter, which are mediated by as yet unexplored versions of type IV filament assembly nanomachines.
We integrate genetic, biochemical and functional analyses with structural, biophysical and modeling approaches to reach detailed mechanistic view of these dynamic macromolecular systems spanning the bacterial envelope. Mechanistic studies of these systems are closely intertwined with more basic questions in protein science, including protein-protein and protein-membrane interactions, the role of ions, protein folding, localization, quality control and turnover.