Inflammation and tissue repair are fundamental processes induced upon damage/insults to ensure protection from potentially harmful agents and restore tissue integrity, a prerequisite for function. Restoring tissue homeostasis requires a tightly regulated crosstalk of different cell types, structures and innate defense mechanisms. Our laboratory is investigating the role of stromal cells, a diverse and abundant population of tissue resident mesenchymal cells that play a dominant role in organ development and physiology. Stromal cells are essential in several biological processes including immune cells recruitment, interaction and survival, angiogenesis, maintenance of stem cells, as well as regeneration of parenchymal, vascular and nerve structures. When not properly regulated, these processes lead to pathological responses. The stromal microenvironment is therefore emerging as an essential player in several chronic diseases characterized by dysregulation of immune responses, including chronic inflammatory/fibrotic diseases, degenerative diseases and cancer.
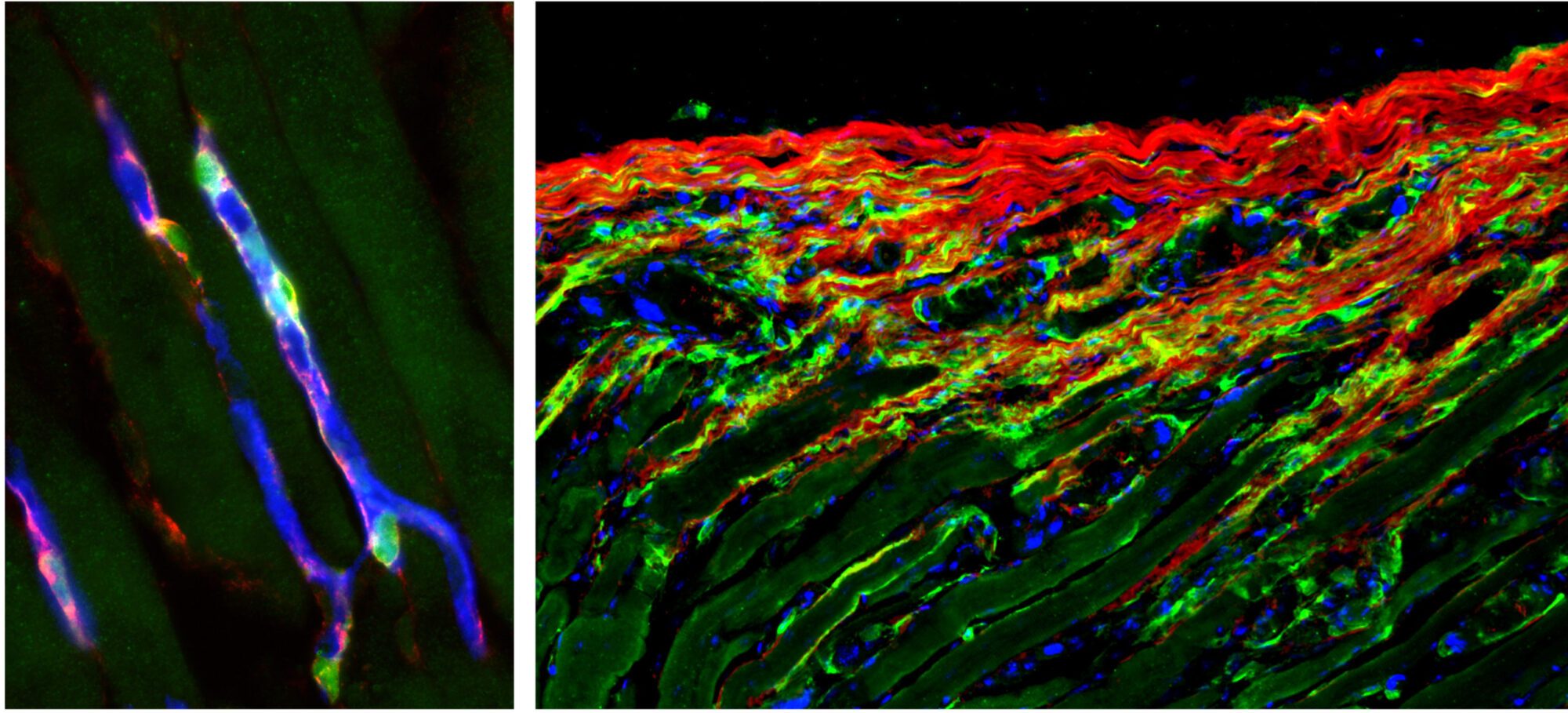
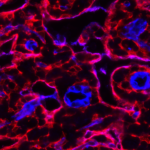
Stromal cells (green) wrapped around blood vessels (blue) in the skeletal muscle are rapidly activated upon injury (left image) and generate matrix overproducing stromal cells during the repair process (right image). Aberrant regulation of the scarring process leads to life-threatening tissue damage and loss of organ function.
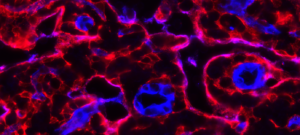
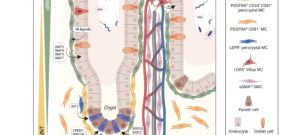
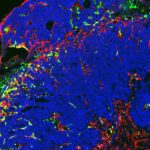
Stromal cells are composed of heterogeneous populations, including common and tissue-specific cell types across organs. Because of the inherent complexity of the system, we address how stromal cells are activated and function in a multidisciplinary manner, with a focus on organs with high remodeling/regenerative capacity such as the skeletal muscle, intestine, skin and adipose tissue. In this context, our work has shown that 1) Stromal mesenchymal cells wrapped around blood vessels are a major source for matrix overproducing fibrotic cells. In solid tumors, they are a major brake to antitumor immunity, 2) Stromal subsets play an essential role in the intestinal stem cells niche and intestinal inflammation, 3) Maturation of intestinal stromal cells in the first few weeks after birth is required for intestinal homeostasis and balanced inflammatory and repair responses upon injury.
Using a range of experimental approaches, including transcriptomics (RNAseq), epigenomics, genetic lineage tracing, depletion models, pharmacological approaches and high resolution cell imaging, our laboratory further explores different aspect of stromal regulation of tissue homeostasis and disease pathogenesis. Our research is currently focusing on:
- The stromal niches in regulation of barrier sites such as the intestine and skin
- Stromal regulation of inflammation and tissue adaptation to environmental inputs
- The stromal tumor microenvironment in tumor immunity and immunotherapies
Join us! We are looking for talented postdocs, PhD students or Master students with an interest in tissue biology/immunity, regeneration or inflammation. If you are interested in joining our lab, please send your CV and a motivation letter to Lucie Peduto (lucie.peduto@pasteur.fr).
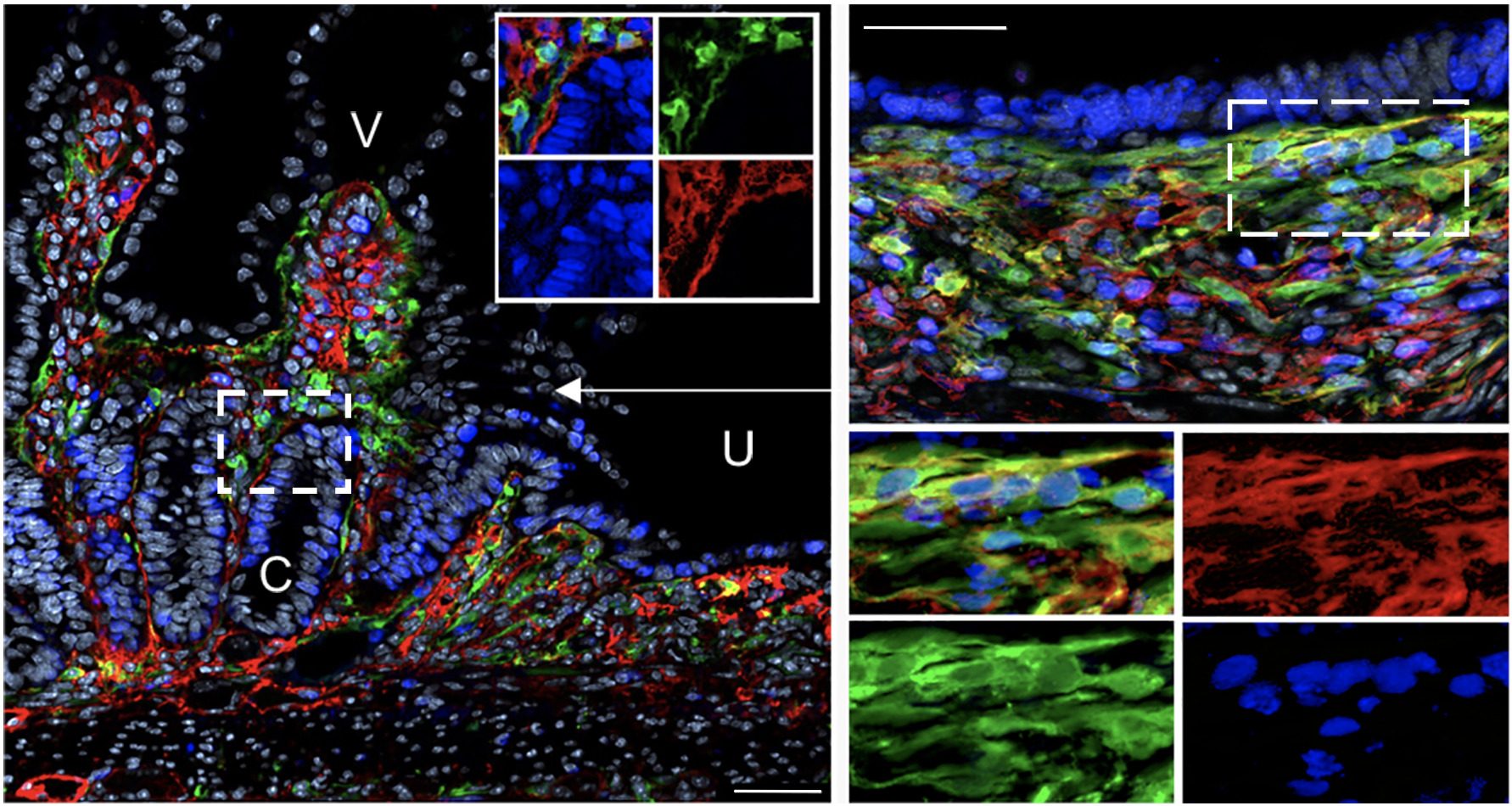
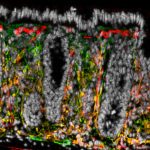
Intestinal stromal cells (green and red) are activated upon mucosal damage induced by nonsteroidal anti-inflammatory drugs and orchestrate intestinal repair and inflammatory responses in cooperation with stem cells and immune cells. V: villi; C: crypts; U: ulcer. (Jacob et al., Cell Stem Cell 2022)
Press release: “Stromal cells, maestros of the intestine”. Read here
Le journal de la recherche: “Découverte, autour de la tumeur, de cellules qui régulent l’immunité antitumorale”/”Discovery of peritumoral stromal cells that regulate antitumor immunity” https://www.pasteur.fr/fr/journal-recherche/actualites/decouverte-autour-tumeur-cellules-qui-regulent-immunite-antitumorale.

Selected publications:
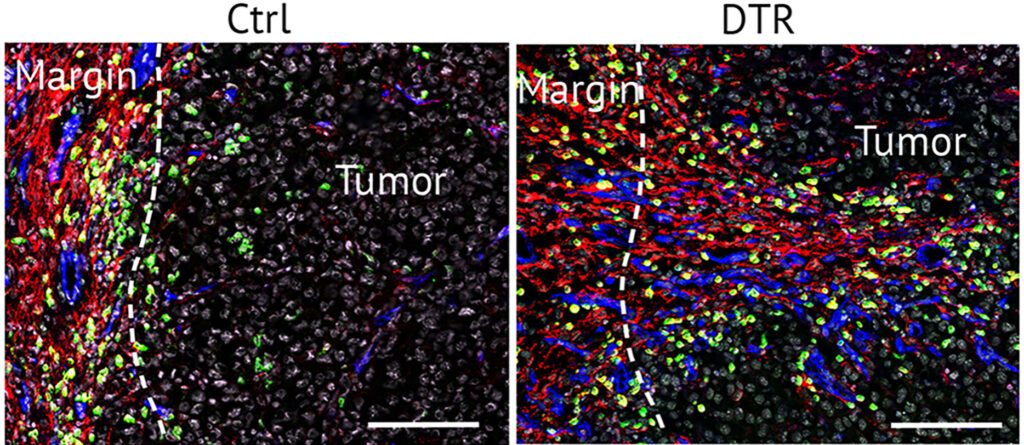
Di Carlo et al. Depletion of slow-cycling PDGFRalpha+ADAM12+ mesenchymal cells promotes antitumor immunity by restricting macrophage efferocytosis. 2023. Nature Immunology, 24(11):1867-1878.
Jacob et al. PDGFRalpha-induced stromal maturation is required to restrain postnatal intestinal epithelial stemness and promote defense mechanisms. 2022. Cell Stem Cell, 29(5): 856-868.
Di Carlo & Peduto. The perivascular origin of pathological fibroblasts. 2018. J Clin Invest, 128(1): 54-63.
Stzepourginski et al. CD34+ mesenchymal cells are a major component of the intestinal stem cell niche at homeostasis and after injury. 2017. PNAS, 114(4): E506-E513.
Dulauroy et al. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. 2012. Nature Medicine, 18(8): 1262-1270.