Intracellular pathogens infect and reproduce within infected cells. A fundamental challenge for these kind of pathogens is to obtain the specific nutrients they need to grow inside infected cells. As intracellular pathogens can only obtain nutrients from the interior of infected cells, modulating the metabolism of host cells to fuel pathogen growth during infection might be an interesting virulence strategy employed by intracellular pathogens to grow intracellularly. On the other hand, it can be hypothesized that host cells could have developed processes to sense pathogen-induced metabolic modifications, which might trigger cell-autonomous immune responses against infection.
Our team is interested in understanding how the metabolic crosstalk between pathogenic bacteria and host cells impacts infection. We use intracellular bacteria as models to study metabolic host-bacteria interactions. Legionella pneumophila and Salmonella enterica ser. Thyphimurium infect and replicate within macrophages during the progress of the human disease (legionellosis and salmonellosis, respectively). Macrophages are innate immune cells in charge of fighting bacterial infection and they reprogram their metabolism upon activation. Thus, the team is studying the metabolic alterations induced by intracellular bacteria on human macrophages, and the impact of these metabolic shifts on the intracellular life of pathogenic bacteria during the course of infection. 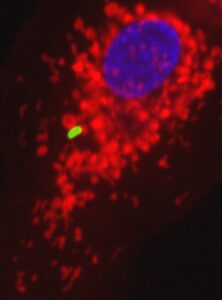
We discovered that: 1) Legionella pneumophila infection increases glycolysis during infection of human primary macrophages; 2) the bacterium injects a bacterial protein called MitF through its type 4 secretion system (T4SS), which induces the fragmentation of mitochondrial networks in the infected cells; 3) Legionella-induced mitochondrial fragmentation leads to a reduction of mitochondrial OXPHOS; 4) Legionella infection thus leads to a high glycolytic and low OXPHOS metabolism in the infected cell that resembles the Warburg metabolism (aerobic glycolysis) found in cancer cells; 5) host glycolysis is important for bacterial replication; 6) in order to avoid cell death of the infected host cell, Legionella maintains mitochondrial membrane potential (mΔψ) in the absence of OXPHOS; 7) to maintain mΔψ, Legionella also injects the T4SS effector LpSpl to induce the ‘reverse mode’ of the mitochondrial FO-F1-ATPase (references here, here and here)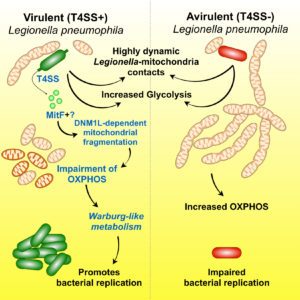
We are now trying to understand: 1) how the OXPHOS machinery is exactly regulated during infection; 2) which are the specific metabolic fluxes altered when intracellular bacteria infect human macrophages; 3) whether Salmonella Thyphimurium also modulate macrophage metabolism and, if is the case, whether the strategies used are similar to those uncovered during the study of Legionella infection; 4) whether metabolic host-bacteria interactions can be used to predict the outcome of bacterial infection; 5) which are the danger-associated metabolic modifications triggered during bacterial infection in macrophages; 6) whether using drugs to target host metabolic pathways could be new host-directed therapeutical strategies to tackle infection by intracellular bacteria.









