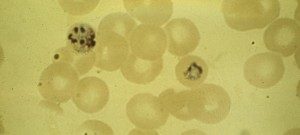
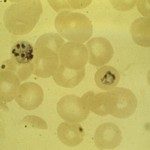
The blood stage of the malaria parasite life cycle is responsible for all the clinical symptoms of malaria. During the blood stage plasmodium merozoites invade and multiply within red blood cells (RBCs). We are interested in understanding the receptor-ligand interactions that mediate the invasion process. Understanding the key receptor-ligand interactions involved in host cell invasion may open new paths for the development of intervention strategies that inhibit red cell invasion and block blood stage parasite growth. We also study the process by which newly developed merozoites in a blood stage schizont egress following complete development.
Parasite proteins involved in red cell invasion and egress: The invasion of red cells by malaria parasites is a complex process that involves a series of specific molecular interactions between parasite proteins and red cell receptors that mediate different steps in the invasion process. We study two families of parasite proteins, namely, the erythrocyte binding antigen family, which includes P. falciparum EBA175 and P. vivax Duffy binding protein (PvDBP), and the P. vivax reticulocyte binding protein family (PvRBPs) and their P. falciparum homologs (PfRH1, PfRH2a/b and PfRH5). Structure function studies are used to map functional receptor-binding sites within these parasite proteins and antibodies to these binding domains are tested for inhibition of P. falciparum growth in vitro individually and in combination to identify parasite antigens that could be included in a blood stage vaccine for P. falciparum malaria.
Signaling pathways involved in regulating red cell invasion and merozoite egress. A number of the parasite proteins that mediate key interactions with host receptors to mediate invasion are initially expressed in internal apical organelles referred to as micronemes and rhoptries and are exported to the apical surface of merozoites during the invasion process. We are interested in identifying the signals and understanding the signaling pathways that regulate the secretion of microneme and rhoptry proteins during invasion. We use molecular genetic, biochemical as well as phosphoproteomic approaches to define the signaling pathways that trigger microneme and rhoptry secretion. We are also interested in understanding the signals and signaling pathways that regulate egress of merozoites from mature schizonts. We have demonstrated that a number of key effectors that play a role in egress are localized in apical organelles such as micronemes and exonemes. The secretion of these organelles is triggered by rise in internal Ca2+. Understanding the signaling pathways that mediate these critical steps in the parasite life cycle can open new avenues for the development of novel targets for drugs that inhibit parasite growth by blocking apical organelle release during egress or invasion.
Malaria vaccine development. We use our understanding of the key receptor-ligand interactions involved in RBC invasion by Plasmodium merozoites for the development of recombinant vaccines that target blood stage parasites. We are developing recombinant vaccines based on functional receptor-binding domains of parasite proteins such as P. vivax Duffy binding protein (PvDBP), P.vivax reticulocyte binding proteins, P. falciparum EBA175 and PfRH5 that mediate critical interactions with host receptors to mediate invasion. Combinations of parasite ligands that yield high rates of growth inhibition due to synergy are identified for development as multi-component vaccine candidates. Efforts are also underway to identify novel delivery strategies that elicit robust immune responses against such multi-component vaccines. These include exploring novel viral vectors, protein conjugation technologies and novel nano-particles formulated with potent adjuvants.