About
Emi Murayama, Anne Schmidt, Doris Lou Demy, Anne-Lou Touret, Léa Torcq, Sara Majello, Catherine Vivier, Mylène Lancino, Mireille Carrère, Charnese Bowes, Ramil Noche
We found that as in mammals, the first leukocytes to arise in the zebrafish embryo are the ‘primitive macrophages’, born in the yolk sac (Figure 1 and movie 1) (Herbomel et al., 1999). From there, these macrophages quickly invade the still unvascularized embryonic tissues, notably the brain and retina where they become microglia – the resident macrophages of the central nervous system (CNS) (Herbomel et al., 2001).
 |
|
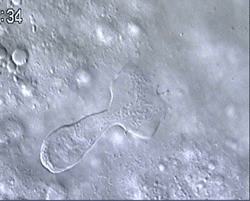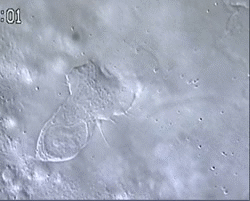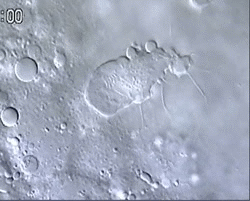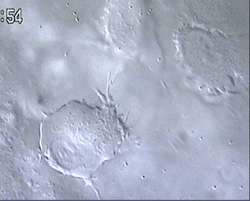
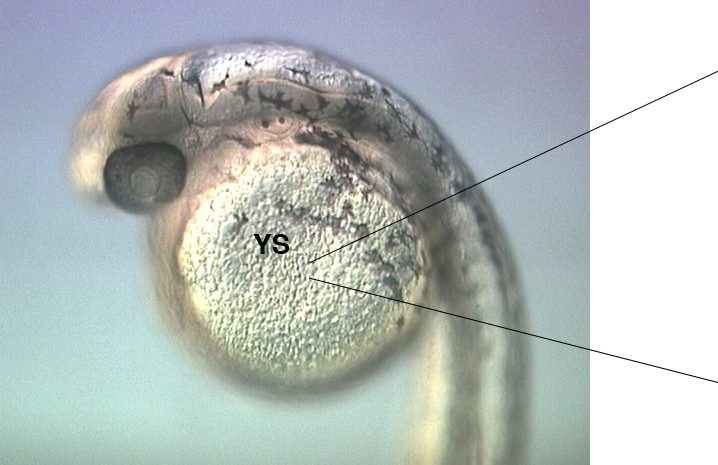
Figure 1 and Movie 1. Left, live zebrafish embryo, 30 hrs post-fertilization (hpf). Primitive macrophages arise from mesoderm cells initially situated just rostral to the heart field, that emigrate to the nearby yolk sac (YS) to differentiate in macrophages by 22-25 hpf. These swiftly moving cells that continually change shapes (Movie 1, video-enhanced DIC/Nomarski microscopy) soon disperse throughout the embryo, especially in the head.
We found that the primitive macrophage progenitor cells actually also give rise to a similar number of neutrophilic granulocytes (neutrophils), that also disperse and then live in the tissues , except the CNS (Le Guyader et al., 2008). We found that even later in development, in juvenile zebrafish as well as in other fish, and frogs, neutrophils are present in healthy, uninflammed tissues, unlike in mammals. Since we found them to be as competent as their mammalian homologues in fighting infections, studying how fish neutrophils live harmlessly in tissues might teach us how to dampen excess inflammation caused by neutrophils in human tissues.
In all vertebrates, after the primitive wave of hematopoiesis, definitive hematopoiesis initiates, with the emergence of hematopoietic stem cells (HSCs), which will generate the full range of leukocyte cell types found in the adult, including the lymphocytes in charge of adaptive immunity. We demonstrated that in zebrafish like in mammals, multipotent HSPCs (hematopoietic stem and progenitor cells) that include the future long-term HSCs initially emerge along the ventral side of the embryo’s central artery, the aorta (Figure 2), and from there undergo a journey through the blood and to successive dedicated niches, where they will give rise to multi-lineage hematopoiesis (Murayama et al., 2006 ; Kissa et al., 2008). We discovered that the first of these niches actually lies in the fish’s tail, around a transient vascular plexus formed by the ramification of the caudal vein, and we named it the Caudal Hematopoietic Tissue (CHT) (Figure 2) (Murayama et al., 2006). Like the fetal liver in mammals, the CHT is a transitional niche for the homing, expansion and differentiation of HSPCs before their migration to the final hematopoietic organs – the thymus and kidney (in fish, adult hematopoiesis takes place not in bone marrow as in mammals, but in ‘kidney marrow’). We could then trace, for the first time in a vertebrate, the routes by which these HSPCs migrate to seed the thymus rudiment and become the first lymphocytes (Kissa et al., 2008).
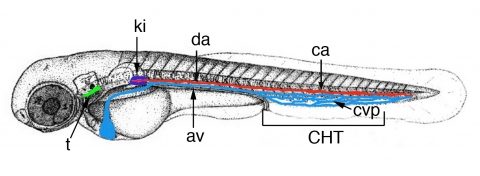
Figure 2. Time-line of zebrafish larval hematopoiesis. Zebrafish embryos become ‘swimming larva’ as they hatch, by 2.5 days post-fertilization (dpf). Definitive hematopoiesis starts in the embryo’s trunk with the transformation of endothelial cells of the dorsal aorta (da) ventral wall into hematopoietic stem/progenitor cells (HSPCs), from 32 to 56 hrs post-fertilization (hpf), and their entry in blood circulation through the axial vein (av). These HSPCs then home to the Caudal Hematopoietic Tissue (CHT) formed around the transient caudal vein plexus (cvp, blue), where they expand and differentiate into several lineages for about a week. From there, some migrate again to seed the thymus rudiment (t) from 56 hpf, then pronephric kidney (ki), the site of adult hematopoiesis, from 4.5 dpf.
We could then resolve a long-standing debate in stem cell biology by demonstrating through high-resolution live imaging that the initial HSPCs that include the long-term HSCs arise directly from the endothelial cells forming the aorta ventral wall, through an unexpected process that does not involve cell division, but a contraction, then strong bending, then egress of single endothelial cells from the aorta ventral wall (floor) and their concomitant transformation into HSPCs (Figure 3, Movie 2). This process, which we named the endothelial hematopoietic transition (EHT), is an entirely novel type of cell transition, by which an epithelial (here endothelial) cell belonging to a thin epithelium can leave it without perturbing its function and become a free cell endowed with a new multipotent identity – a HSPC. We also showed that the runx1 gene, previously known to be essential for HSPC emergence in all vertebrates examined, is actually required for the EHT to occur successfully (Kissa et al., 2010).
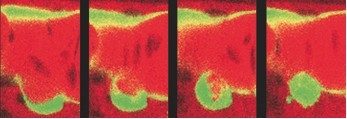
Figure 3 (from left to right) : successive views over 5 hrs of a vascular endothelial cell (bottom green cell) of the aorta floor transforming into a hematopoietic stem/progenitor cell.
More recently, we further characterized this unprecedented cellular transition through in vivo imaging and analyses of the dynamics of subcellular components such as cell junctions and acto-myosin cytoskeleton. This led to a first working model of how the EHT is accomplished, in the cellular and biomechanical context of an artery in which blood flows steadily (Lancino et al., 2018).
In parallel, we have been deepening our study of the first niche in which the aorta-derived HSPCs home, the caudal hematopoietic tissue (CHT). To this end, we have isolated zebrafish mutants in which HSPCs emerge from the aorta and home to the CHT, but then are uncapable to survive and expand there. We found that the CHT niche consists in two components, a venous plexus that traps circulating HSPCs, and a network of stromal reticular cells, that surprisingly derive from the caudal somites overlying the presumptive CHT, and are necessary for the maintenance, expansion and multi-lineage differentiation of HSPCs in the niche (Murayama et al., 2015).
Finally, as an example of a tissue resident macrophage population, the microglia of the CNS is especially interesting given its involvement in CNS homeostasis and neurodegenerative diseases. We have characterized zebrafish mutants specifically devoid of microglia, in which macrophages disseminate through the developing embryo except in the CNS, and we analyse the causes of this defect, according to the mutated gene (Demy et al., 2017). These mutants should also constitute valuable models for neurobiologists to study the consequences of microglia absence on brain maturation.