SITE UNDER CONSTRUCTION (expect changes)
In the last 5 years, interest in cryo-electron microscopy (cryo-EM) has grown exponentially due to groundbreaking new developments in instrumentation. This has resulted in the 2017 Nobel Prize in chemistry being awarded to three pioneers from the field of cryo-EM. Institut Pasteur has invested significantly in this technology with the purchase of 4 high-end systems and the erection of the Nano-Imaging Core Facility.
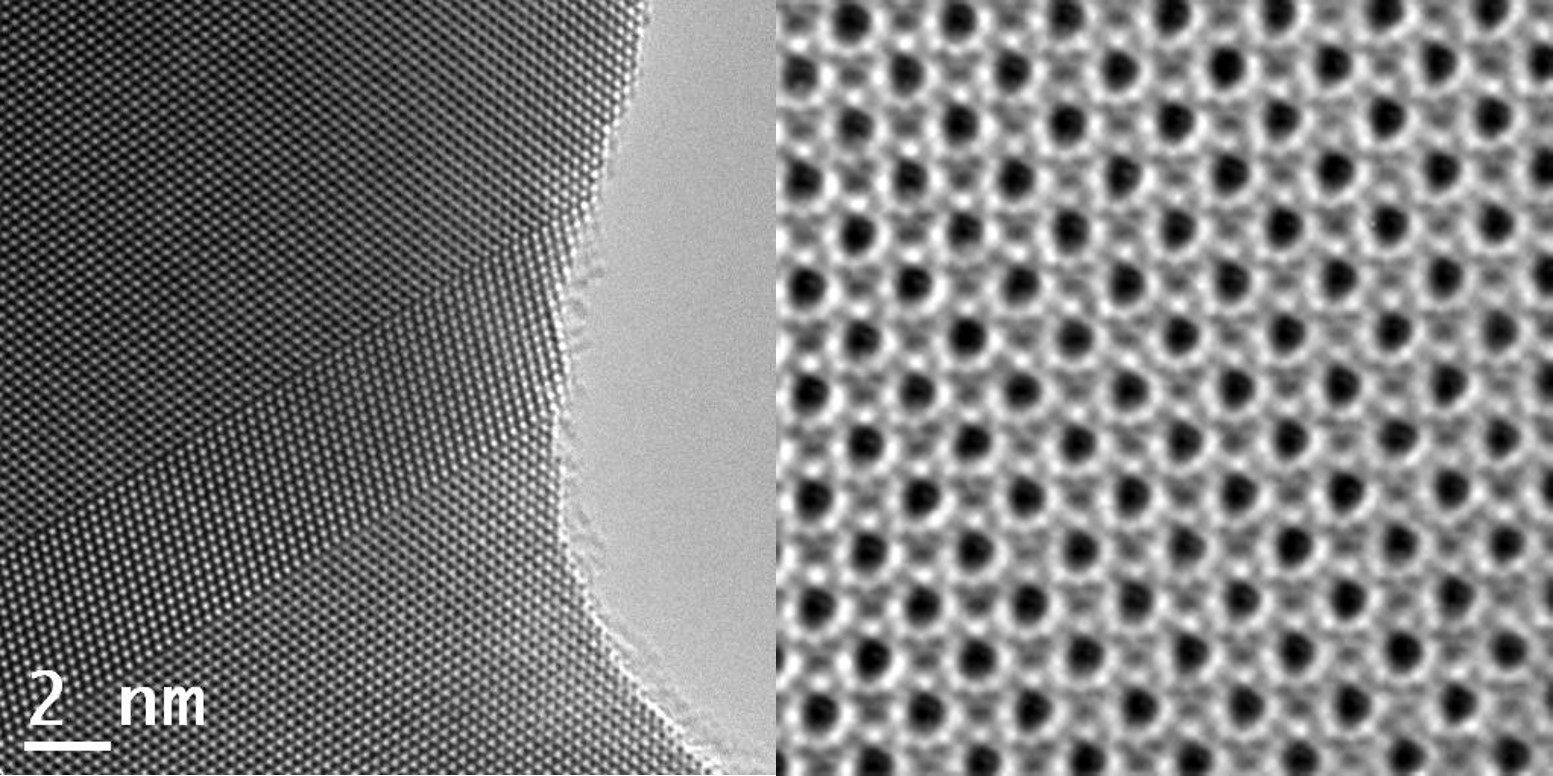
Transmission Electron Microscopes are known for being able to achieve atomic resolution in material science applications (MS). This is possible due to the well defined nature of the specimens that are in many cases crystalline (i.e. silicon wavers) or adopt other latices structures (i.e. metals). In addition the elements under scrutiny often fall in the category of heavy elements with large nuclei that provide additional forms of contrast in an electron microscope. On top of this, these samples can withstand a high dose of electrons before being destroyed by the intensity of the beam, which provides a high signal-to-noise-ratio (SNR) in the recorded images. As a result, MS samples can often achieve very high sub-Ångström resolution in the images that allow clear images of individual atoms.
In life science (LS) we use electron microscopes to study cells, organelles, macro-molecular machinery and proteins inside or isolated outside the environment of the cell. Proteins are almost never crystalline in nature, nor are they well order or well defined. They are very long polymeric molecules folded into flexible (at the atomic level) structures consisting of predominantly light elements (C, O, N, H). The atomic bonds in these molecules can be easily broken by the high energy electrons which will deform the protein structure rapidly. As a consequence, images need to be recorded using a significantly lower dose, resulting in noisy images that have additionally low contrast due to the light elements and the absence of lattice order. Only by developing and using special dedicated low noise cameras and new image processing algorithms in the last few years, has it been possible to extract high resolution information on a relative routine basis.
The core facility will focus on 4 main applications in cryo-EM: Sample Screening, Single Particle Analysis (SPA), cryo-Electron Tomography (CET) and cryo-correlative light and electron microscopy (cryo-CLEM)
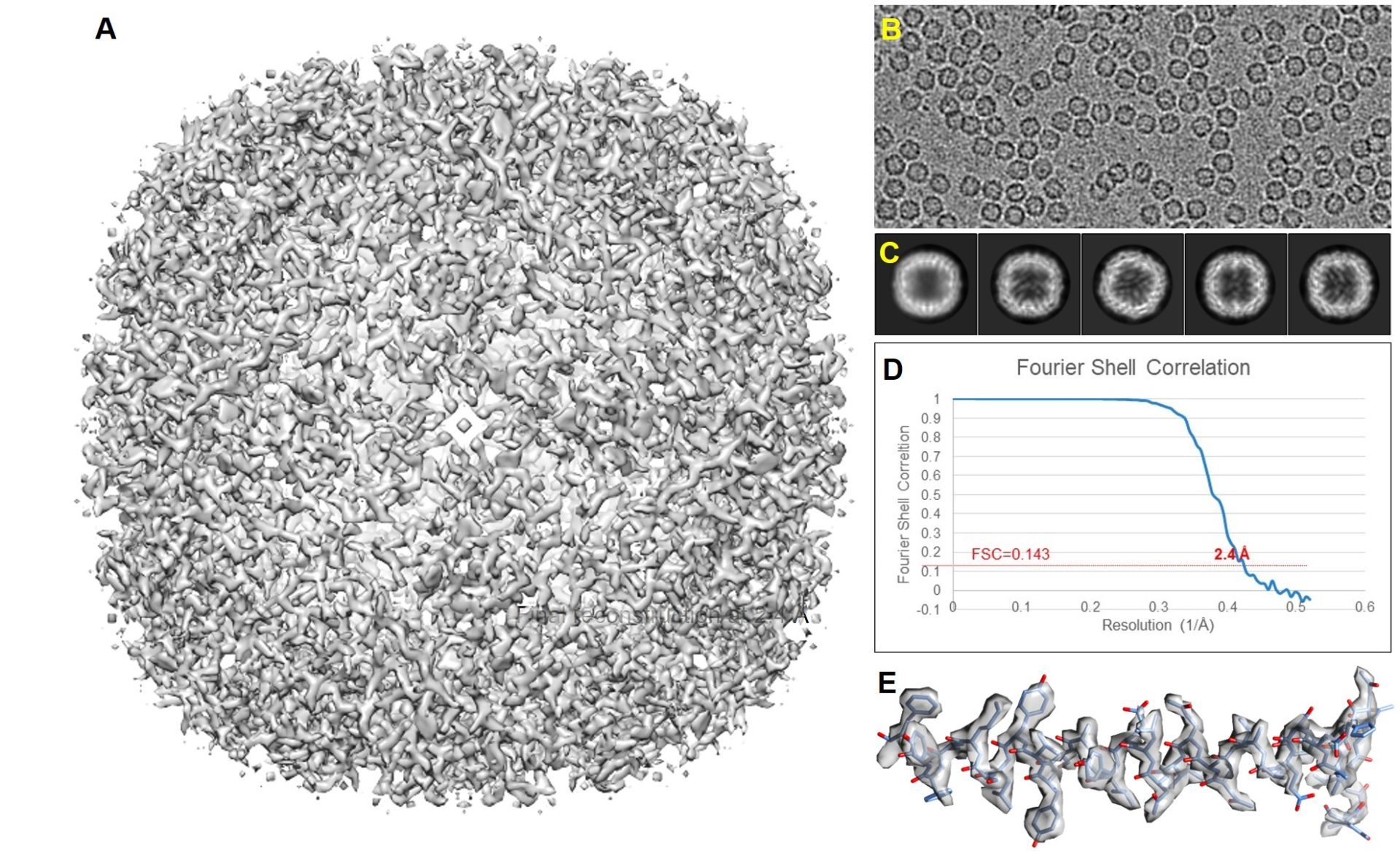
Single Particle Analysis (SPA)
Cryo-FIB and Tomography