The Flow Cytometry Core Facility of the Institut Pasteur, provides cutting-edge instrumentation, as well as high-level scientific and technical expertise in flow cytometry in general. The core facility is part of the C2RT (Center for Technological Resources and Research), a center gathering the Technology and and the technological core facilities of the campus.
Who we are? What we do

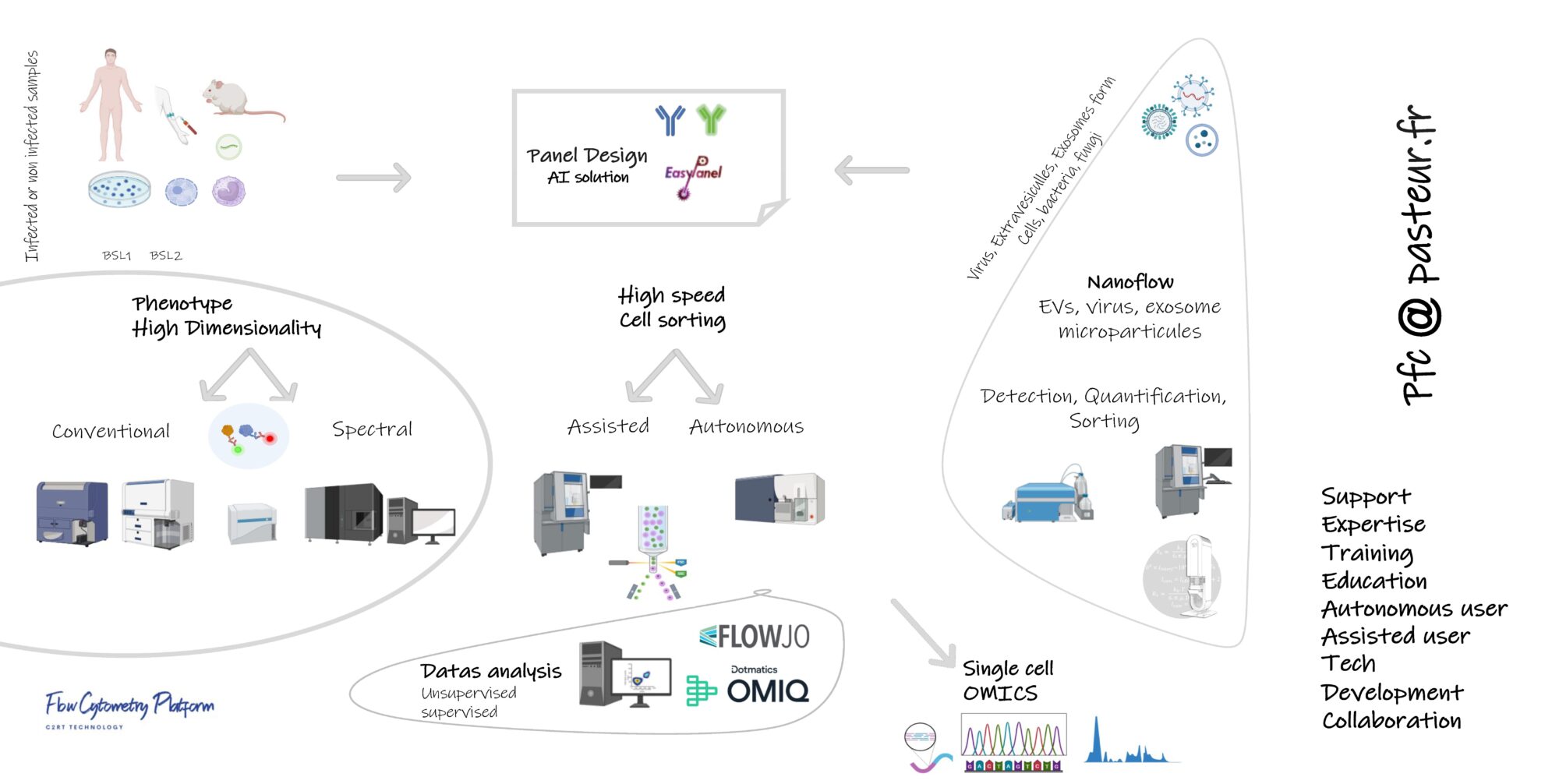
Technological pipelines & New
♦ High dimensional cytometry pipeline (conventional and spectral Cytometry):Choosing the best marker/dye-combination can make all the difference to your flow cytometry panels. But that selection can sometimes feel more art than science. Our flow cytometry experts can help you find the right balance between epitope expression and fluorochrome brightness, ensuring you’ve got the very best configuration for your application of interest – whatever it may be.
♦ Nanocytometry pipeline : subcellular analysis and nano sorting (Nano FCM, MoFlow Astrios).
♦ Unsupervised data analysis (FlowJo plugins & OMIQ)
♦ Single cell phenotyping and cell sorting are supported by the Cytometry platform.
The scBiomarkers UTechS of the Institut Pasteur proposes numerous resources for single cell analysis, open to the entire academic and clinical research community.
Service & Support
♦ The platform provides services on cell sorting, flow cytometry analysis, consulting, data analysis, troubleshooting, and training.
♦ In our core facility, equipment is completely state-of-the-art with :
∗ 7 high-speed cell sorters
∗ 5 high-end analyzers : (Conventional cytometry & Spectral cytometry)
∗ 1 NanoAnalyser
∗ 1 instrument for cell enrichment by magnetic beads : AutoMACS Pro Separator
∗ 1 VideoDrop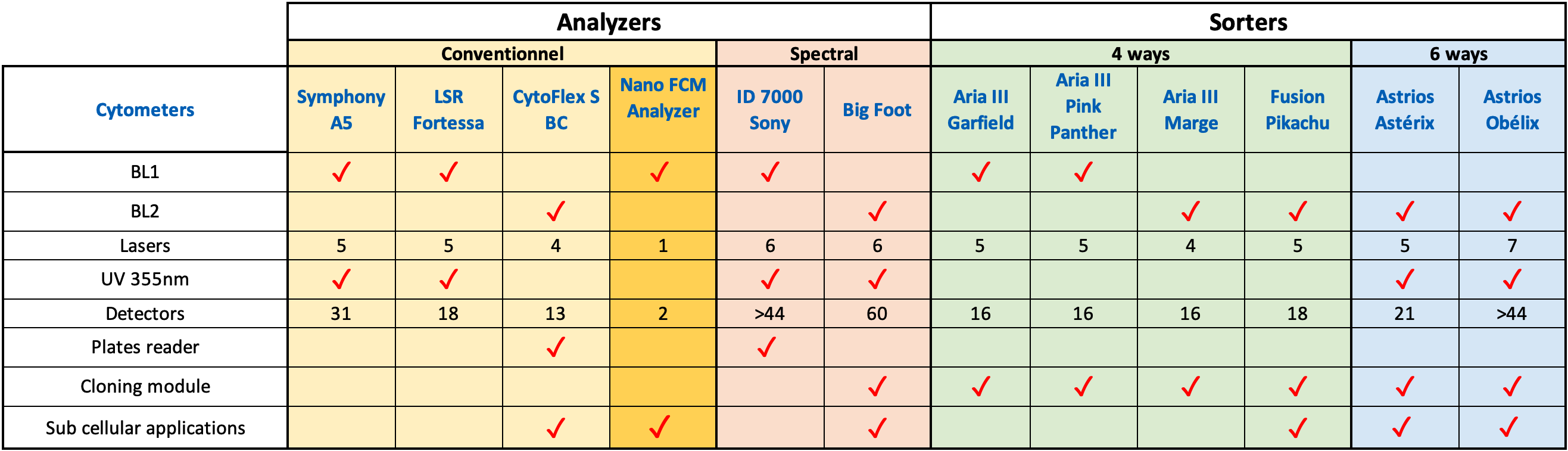
♦ We also gather resources for our users : Cytometry aids and protocols.
♦ We support two software packages for flow cytometry analysis. Both software packages are available as site licenses through individual internet logins and Virtual Machine ( send an email at FlowJo-VM@pasteur.fr)
∗ FlowJo
∗ Kaluza
∗ OMIQ
Training (see :PFC specific training)
Training courses proposed by the flow cytometry core facility are available for Institut Pasteur researchers, as well as external users (academic and industrial).
♦ First, we give courses on the conceptual aspects of flow cytometry (face-to-face or online), then we give practical training on specific instruments.
♦ We propose FlowJO™ , OMIQ and Kaluza trainings to teach researchers how to analyze flow cytometry data in an efficient way. In FlowJo X plugins and OMIQ can be used for unsupervised analysis
♦ Every year, we also propose a one week Flow Cytometry Course based on “Fundamental Cytometry”.
Quality management![]() As we are involved in quality management (ISO9001), we are attentive to the expectations of users. We do not focus only on the technical aspects of the instrumentation, but we make the link between these technical aspects and the scientific questions.
As we are involved in quality management (ISO9001), we are attentive to the expectations of users. We do not focus only on the technical aspects of the instrumentation, but we make the link between these technical aspects and the scientific questions.
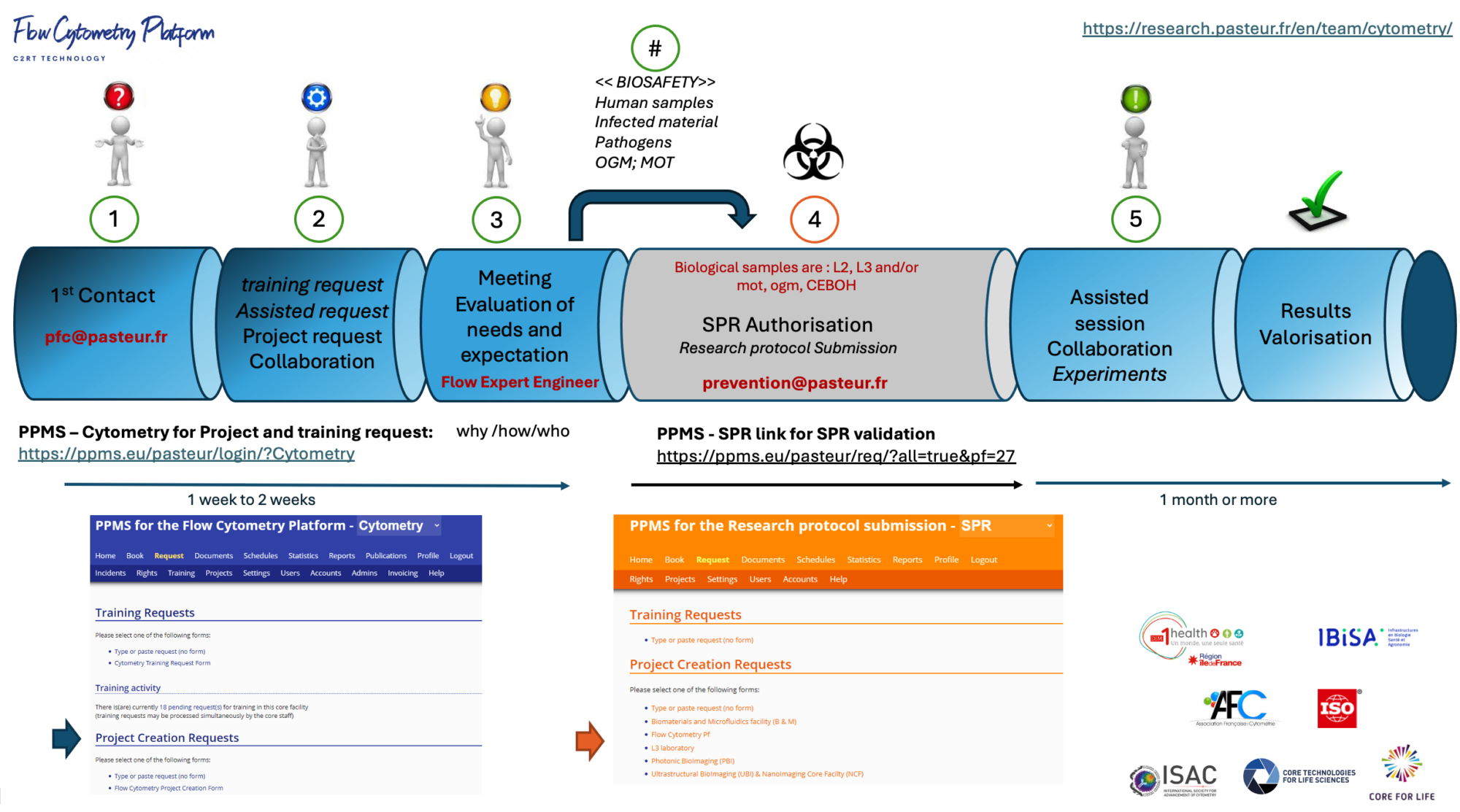
HOW TO ACCESS : The Cytometry Platform is an open-access facility that welcomes projects and teams from the Institut Pasteur, from the International Network of the Institut Pasteur, from academia, hospitals and industry. The Cytometry platform uses Stratocore Platform Pilot Management System (PPMS) as the booking system. Once your project is submitted you can book an Instrument/training (PPMS).