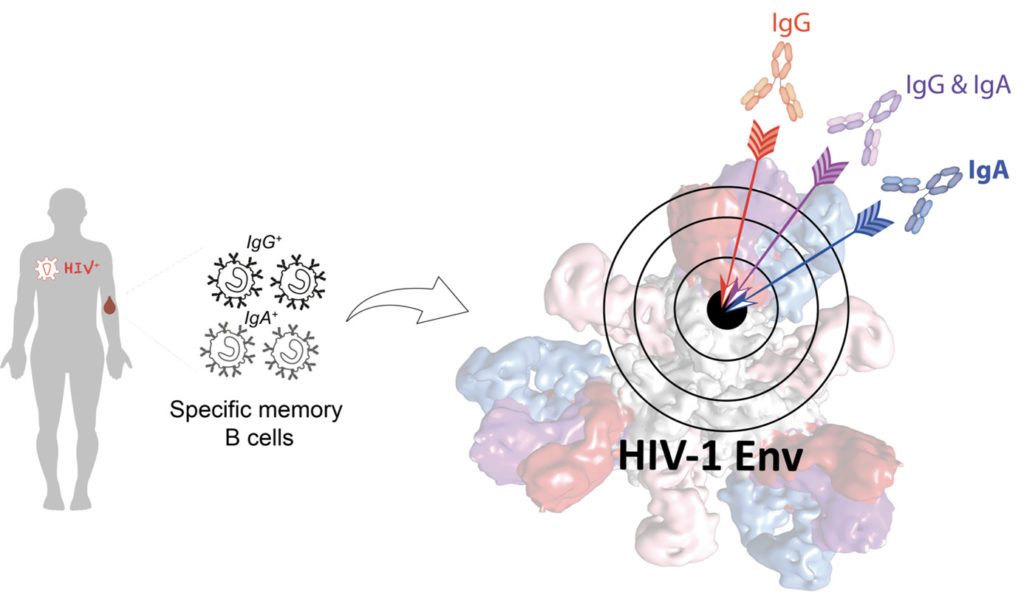
Broadly neutralizing antibodies (bNAbs) develop in rare HIV-1 infected humans, and neutralize a large panel of viral quasi-species. Over the past decade, hundreds of bNAbs have been isolated, some of which can protect non-human primates from infection. Hence, bNAbs hold great promises for HIV-1 treatment and prevention by vaccination or passive immunoprophylaxis. Indeed, decrypting the B cell ontogeny of HIV-1 bNAbs is paramount for vaccine design. In this study, we describe the detailed characterization of IgA and IgG bNAbs from three distinct B cell lineages in a viremic controller, two of which comprised only IgG+ or IgA+ blood memory B cells; the third combined both IgG and IgA clonal variants. 7-269 bNAb in the IgA-only lineage displayed the highest neutralizing capacity despite limited somatic mutation, and delayed viral rebound in humanized mice. bNAbs in all three lineages targeted the N332 glycan supersite. The 2.8 Å-resolution cryo-EM structure of 7-269-BG505 SOSIP.664 complex showed a similar pose as 2G12, on an epitope mainly composed of sugar residues comprising the N332 and N295 glycans. Binding and cryo-EM structural analyses showed that antibodies from the two other lineages interact mostly with glycans N332 and N386. Thus, multiple B cell lineages of IgG and IgA bNAbs focused on a unique HIV-1 site of vulnerability can codevelop in HIV-1 viremic controllers. Also, potent IgA bNAbs to the N332 supersite are elicited in response to HIV-1 infection, with an affinity maturation signature compatible with the one induced by vaccines, which could be essential for blocking HIV-1 mucosal transmission.
Source
Epitope convergence of broadly HIV-1 neutralizing IgA and IgG antibody lineages in a viremic controller, Journal of Experimental Medicine, March 1, 2022