Since the beginning of my career, my goal has been to understand the mechanisms of effector immune responses to viruses and the related viral countermeasures. During my thesis, I studied the origin of type I interferons (IFN-I) and the impact of CD8 T cells on viral control in an in vivo model of human immunodeficiency virus-1 (HIV-1) infection. During my post-doctoral training, my aim was to identify molecular and cellular immune mechanisms controlling viral replication. I rapidly focused my work on the antiviral activities of antibodies. I studied the non-neutralizing functions of anti-HIV-1 broadly neutralizing antibodies (bNAbs) and the mode of action of anti-SARS-CoV-2 antibodies. In 2019, I joined the laboratory of Tineke Cantaert in Institut Pasteur Cambodia as visiting scientist.
My goal is now to combine both basic and translational approaches to tackle novel questions on the mechanisms of action of antibodies against HIV-1, SARS-CoV-2 and emerging viruses.
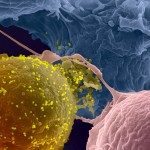
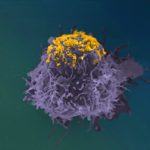
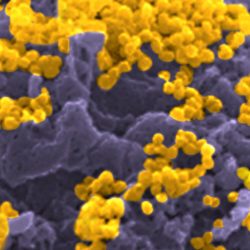
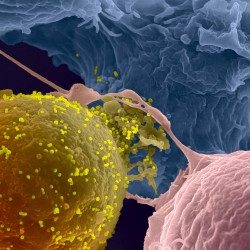
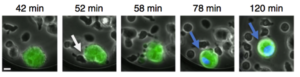
Example of HIV-1-infected cell killing by NK cells: An HIV-1-infected cell (green) and NK cell (smaller dark cells) are trapped in a microwells and recorded. A picture is taken every 5 minutes. A blue dye reveals the dying cell