Lien vers Pubmed [PMID] – 36748301
Lien DOI – 10.1111/febs.16746
FEBS J 2023 Feb; ():
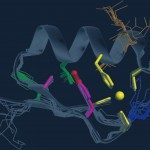
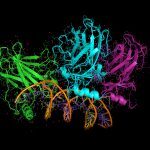
In human cells, de novo purine nucleotide biosynthesis is known to be regulated through the formation of a metabolon called purinosome. Here, we employed a bacterial two-hybrid approach to characterize the protein-protein interactions network among the corresponding enzymes of Escherichia coli. Our study revealed a dense network of binary interactions that connect most purine nucleotide biosynthesis enzymes. Notably, PurK, an exclusive prokaryotic enzyme, appears as one of the central hubs of this network. We further showed that modifications in PurK, which disrupted several interactions in the network, affected the purine nucleotide pools and altered the bacterial fitness. Our data suggest that the bacterial de novo purine nucleotide biosynthesis enzymes can assemble in a supramolecular complex and that proper interactions among the components of this complex can contribute to bacterial fitness.