Présentation
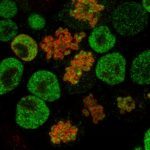
Self-renewing ES cells display dramatic cell-to-cell variability in the expression level of key transcription factors such as Nanog. Whereas Nanog-positive cells self-renew efficiently, Nanog-negative cells are prone to differentiate. Although these two populations can dynamically inter-convert, very little is known about the underlying mechanisms. It is believed that Nanog fluctuations result from stochastic modifications of the activity of the pluripotency network, which is inhibited by Erk and Gsk3b signalling pathways. However, the Nanog-negative state can be inherited during several cell divisions. Thus, yet to describe epigenetic mechanisms may be involved in Nanog heterogeneity. We are currently exploring this possibility and have shown that a histone mark associated with gene repression is enriched at Nanog in undifferentiated ES cells. This enrichment is maintained during mitosis and lost upon inhibition of Erk and Gsk3b, correlating with the loss of Nanog-negative cells. This indicates that the mark that we have identified may be instrumental to epigenetically maintain the negative state.