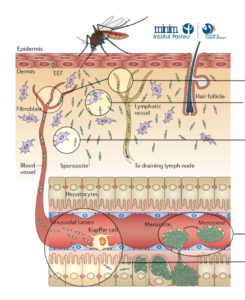
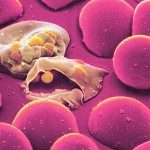
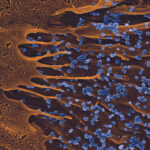
We have been using in vivo imaging and rodent malaria models to better understand the initial steps of malaria parasite infection in the tissues of the mammalian host. Imaging revealed novel steps in the parasite lifecycle in the skin and liver, the role of cell traversal activity in the progression of sporozoites through these tissues and how the parasite crosses the liver sinusoids avoiding the clearance by hepatic macrophages.
Skin phase of malaria infection
blood & lymph vessel invasion, search strategy to locate hotspots of intravasation
cell traversal in the dermis (1) (2)
Kupffer cell evasion and crossing of liver sinusoids
Our research group is now interested in identifying specific determinants of host infection by plasmodial pre-erythrocytic (PE) stages, focused on the key factors involved in the elimination of these stages by the host immune system. We ultimately aim at the development of a modular, chimeric, multi-antigenic pre-clinical PE vaccine capable of sterilizing sporozoite infection. To achieve these goals our research program is based on three complementary axis of research briefly described below:
1. Determinants of host infection by malaria sporozoites
The first axis of our research program aims at scrutinizing the molecular determinants of infection in the skin and liver of the mammalian host. By using imaging, genetic, immunological and pharmacological approaches we intend to identify and characterize the molecules involved in three critical steps of sporozoite lifecycle: (a) the activation of motility in the dermis, (b) the search and subsequent invasion of cutaneous blood vessels, and (c) the specific arrest of parasites in the liver (in collaboration with the laboratory of Joana Tavares, IBMC, PT). Potential targets of neutralizing antibodies will be tested in the axis 3.
2. Determinants of host protection against PE stages
The second axis aims at the identification and characterization of antibodies and immune cells that can efficiently eliminate sporozoites and liver-stages in vivo. Recently, in collaboration with the laboratory of Silvia Boscardin (USP, BR), we discovered that antibodies directed against the central repetitive region of the major surface protein of sporozoites, the circumsporozoite protein, directly kill P. yoelii, P. berghei and P. falciparum sporozoites without the necessity of downstream host effectors. These cytotoxic effectors are mainly associated with the neutralization of sporozoites in the host skin, but high-affinity and high-cytotoxicity antibodies also neutralize sporozoites in the liver. In collaboration with Robert Seder (NIH, US), we are now assessing the correlation between cytotoxicity and protection in vivo using libraries of anti-P. falciparum CSP human monoclonal antibodies.
Regarding the elimination of infected hepatocytes by CD8+ T cells, in collaboration with the laboratories of Fidel Zavala (JHMRI, US) and Katsuyuki Yui (Nagasaki University, JP), we discovered that antigen specific CD8+ T cells recruit other specific and non-specific immune cells to eliminate liver-stages by forming an antigen-driven inflammatory infiltrate around infected hepatocytes. The protective role of non-specific cells recruited to these cellular foci is being assessed, and tested as a complementary way to enhance protection. The generation of these optimal protective effectors is also driving the design of antigens in the axis 3.
3. Pre-clinical development of a multi-antigenic PE vaccine
The third axis of our research program aims at developing chimeric and modular antigens composed by the fusion of protective domains of multiple protective antigens conserved among plasmodial species. In collaboration with the laboratory of Pierre Charneau (Institut Pasteur) we are using lentiviral delivery to identify protective antigens/ domains in vivo and combine them as individual antigens or chimeric fusions. So far, we have identified 8 conserved protective antigens, which when combined sterile protects the vast majority of immunized mice against P. berghei sporozoite challenge. In collaboration with Georges Snounou and Roger LeGrand (CEA, FR), the protective activity of these antigens/ fusions will be assessed using a non-human primate infecting plasmodial species, before ultimately being tested in humans.