Link to Pubmed [PMID] – 19854204
J. Mol. Biol. 2010 Jan;395(1):89-104
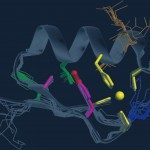
NEMO is an integral part of the IkappaB kinase complex and serves as a molecular switch by which the NF-kappaB signaling pathway can be regulated. Oligomerization and polyubiquitin (poly-Ub) binding, mediated through the regulatory CC2-LZ domain, were shown to be key features governing NEMO function, but the relationship between these two activities remains unclear. In this study, we solved the structure of this domain in complex with a designed ankyrin repeat protein, which helps its crystallization. We generated several NEMO mutants in this domain, including those associated with human diseases incontinentia pigmenti and immunodeficiency with or without anhidrotic ectodermal dysplasia. Analytical ultracentrifugation and thermal denaturation experiments were used to evaluate the dimerization properties of these mutants. A fluorescence-based assay was developed, as well, to quantify the interaction to monoubiquitin and poly-Ub chains. Moreover, the effect of these mutations was investigated for the full-length protein. We show that a proper folding of the ubiquitin-binding domain, termed NOA/UBAN/NUB, into a stable coiled-coil dimer is required but not sufficient for efficient interaction with poly-Ub. In addition, we show that binding to poly-Ub and, to a lesser extent, to monoubiquitin increases the stability of the NOA coiled-coil dimer. Collectively, these data provide structural insights into how several pathological mutations within and outside of the CC2-LZ’s NOA ubiquitin binding site affect IkappaB kinase activation in the NF-kappaB signaling pathway.