A number of infectious agents, including emerging pathogens and agents responsible for nosocomial infections, reach the blood during infection leading to septicemia and meningitis. Despite antibiotic therapy these infections result in severe sequelae and high death rates. A better understanding of the mechanisms of disease is a necessary step for the identification of innovative treatments.
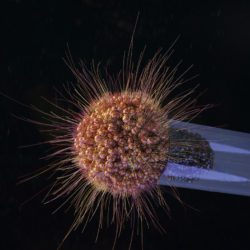
We study the pathogenesis of Neisseria meningitidis (or meningococcus), a Gram-negative bacterium that recapitulates these different pathological effects. This bacterium asymptomatically colonizes the human nasopharynx and pathology is initiated when the bacterium crosses the nasopharynx epithelium and reaches the bloodstream where they survive and proliferate.
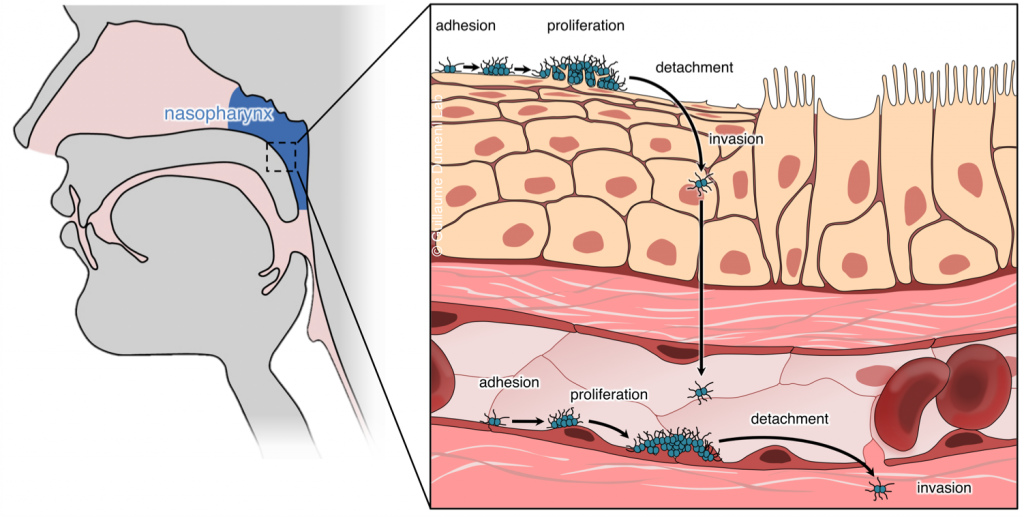
Outstanding questions in terms of understanding N. meningitidis pathogenesis include: how do bacteria cross the epithelium and reach the bloodstream? How do they survive in the blood? How do they damage vessels and reach the cerebrospinal fluid (i.e. cause septic shock and meningitis)? These questions open broader studies regarding tissue biology, bacterial adaptation to different environments and basic functions of the innate immune system. We address these questions at different scales.
- Structural biology of bacterial virulence factors. We study in particular type IV pili and the molecular machinery necessary for their dynamic assembly and disassembly which allow bacteria to adhere host cells and are essential for virulence. We take advantage of, mass spectrometry optimized cross-linking, single particle Cryo-Electron Microscopy and Cryo-Electron Tomography approaches.
- Interaction of bacteria with host cells. Neisseria spp. are extracellular bacteria which manipulate host cell functions from the outside. We study how they mediate this intricate cross-talk with human endothelial cells in culture using fluorescence video microscopy and Cryo-Electron Tomography. We use 2D cultures and 3D microfluidics-based vessel-on-a-chip set-ups to study the impact at the tissue level. This leads us to basic cell biology and mechanobiology questions.
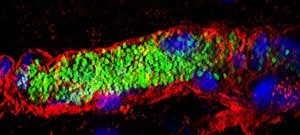
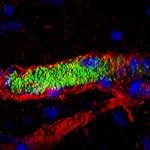
- Impact of infection on blood vessel function. We have developed an animal model based on the xenograft of human skin onto immunodeficient mice. Because of its strict human specificity N. meningitidis type IV pili bind only to capillaries present in the human tissue. Availability of such a model of infection recapitulating the cardinal features of the blood phase of the infection now allows us to address key questions in terms of the bacterial and host processes involved in N. meningitidis-caused vascular damage.
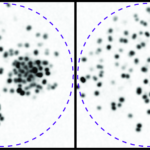
- Innate immune system. Our humanized animal model also allows us to address how the innate immune system reacts to the intravascular infections and also how bacteria evade the immune system.
We thus follow a global and multidisciplinary approach combining microbiology with cell biology, vascular biology, chemistry and physics to study the pathogenesis of N. meningitidis infection.
Whenever possible our basic findings are exploited to design preventive and therapeutic approaches in collaboration with pharmacological companies. It is also essential to us to apply our understanding of N. meningitidis to the study of other bacterial pathogens involved in similar pathologies.