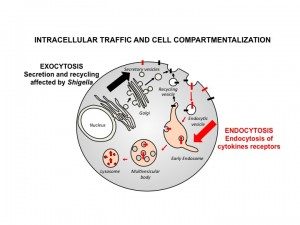
Eukaryotic cells contain extensive internal membranes defining many compartments having each specific functions. However these organelles are constantly moving and reshaping, still retaining their identity. The maintenance of cell compartmentalization in eukaryotes is thus very complex and tightly controlled. Our team studies the intracellular trafficking of eukaryotic cells and its impact on the organization of the cell and tissues. We study in particular the pathways of entry into the cell (endocytosis) and exit pathways (secretion) and their links to plasma membrane composition, cell compartmentalization but also with intercellular communication (immunity) and host-pathogen interactions.
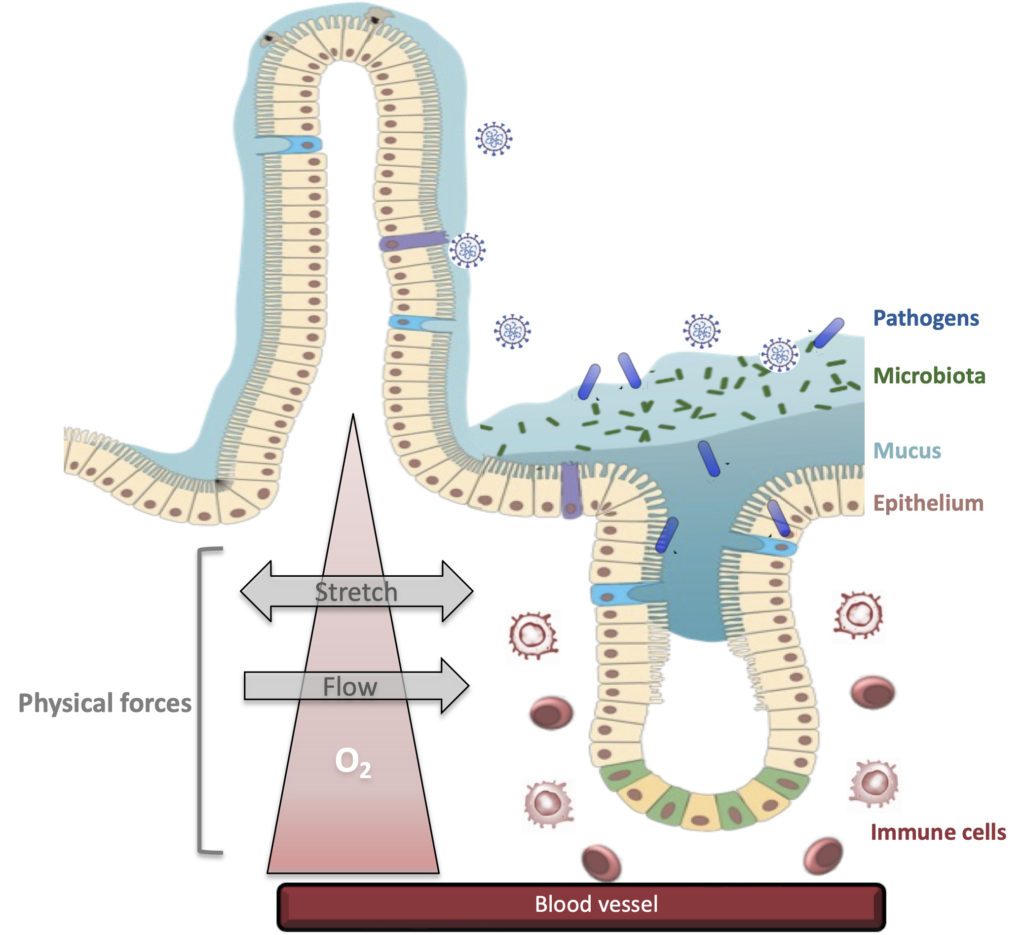
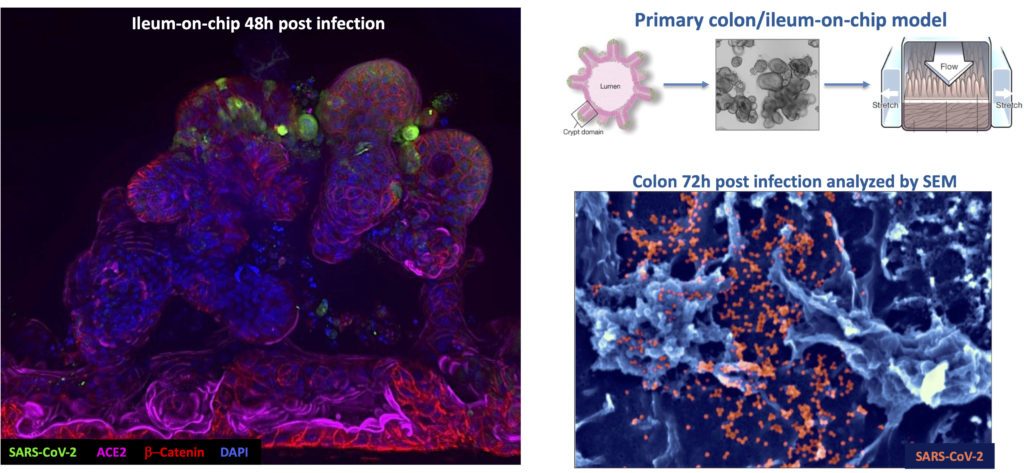
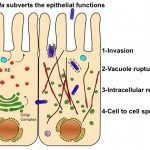
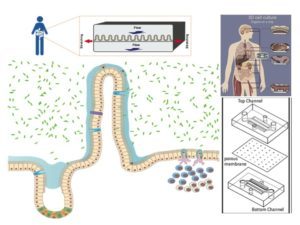
The target organ of our studies is the human intestine composed of multiple cells organized in 3D, acting as a tight barrier between the outside and the inside of an individual. This tissue is permanently exposed to microorganisms some being beneficial (microbiota) but others are detrimental like pathogens. The gut is also constantly subjected to physical forces such as variable oxygen pressure, fluid flow and peristalsis (stretching force). These parameters seem necessary for the organization of this tissue, so part of our work focuses on the role of the mechanical forces of the intestine on the integrity of this barrier. Our recent work using gut-on-chip technology, shows that the mechanical forces of the gut (particularly peristalsis) increases the effectiveness of intestinal infection by the bacterium Shigella (agent of bacillary dysentery).
Our team uses a set of techniques ranging from high resolution microscopy, single molecule tracking, robust image analysis, statistical classification, CrispR-CAS9 genetic editing, organoid to organ-on-chip