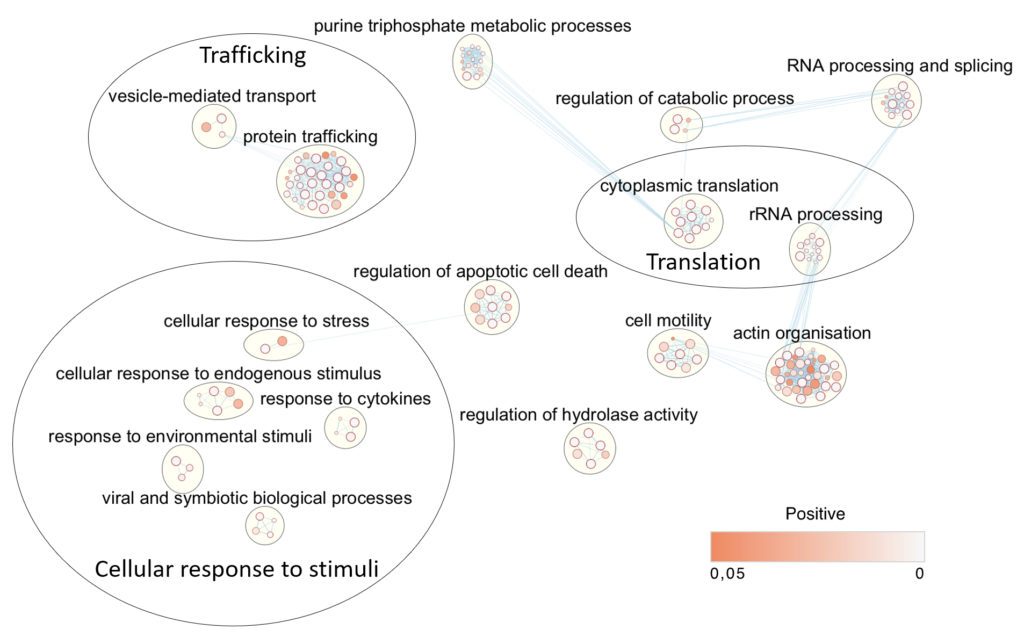
The main focus of the group is to understand the mechanisms of host cell subversion by Leishmania through the release of parasite virulence factors via extracellular vesicles (EVs). A common feature of the most successful intracellular pathogens is the efficient manipulation of their host cell; this is true for bacteria such as Mycobacterium as well as for eukaryotic parasites such as Leishmania. The manipulation of the host, a.k.a. the macrophage, by Leishmania occurs mostly by exporting proteins into the host cell, either as free proteins or inside vesicles. To better understand host-Leishmania interactions, it is crucial to study these secreted proteins. As a signalling kinase released via EVs, Leishmania Casein Kinase 1 (L-CK1.2) is the perfect candidate to understand how pathogens, through vesicular proteins, modify their host as it might be a key regulator of host cell signalling. We showed that L-CK1.2 is closely related to human CK1, essential for intracellular parasite survival and might have been evolutionary selected to interact with and phosphorylate host proteins to modulate macrophage functions. We are currently deciphering the functions of L-CK1.2 in the parasite, particularly the mechanisms responsible for its export, through the study of L-CK1.2 interacting partners; but our main focus is to understand its role in the mammalian host. To this end, we are combining imaging, system-level analyses, gene editing and tool development to study the impact of L-CK1.2 in the context of the macrophage without the interference of the other secreted parasitic proteins to determine its involvement in host subversion.
The group is also involved in the development of novel anti-leishmanial strategies, such as targeting released parasite virulence factors or host proteins interacting with these virulence factors, to restore the host cell anti-microbial potential. We setup a collaboration with Nantes University for chemistry and Paris-Saclay University for in vivo study and identified CTN1122 as an efficient inhibitor of L-CK1.2 with a good Selectivity Index (SI) towards MRC5 cells (52.5 for L. major and 15.5 for L. donovani). We confirmed the specificity of CTN1122 and its anti-leishmanial activity on promastigotes and intracellular amastigotes in vitro and in vivo. These data validate CNT1122 as a lead compound and we are currently optimizing this compound towards preclinical trial.