Advancing Sensory Precision Medicine: From Disease Mechanisms to Therapeutic Solutions
Hearing, balance, and vision are essential for how we experience, interpret, and interact with the world. Yet, sensory impairments—often invisible and progressive—affect millions, profoundly impacting quality of life. Globally, over 460 million people live with disabling hearing loss, a figure projected to exceed one billion by 2050. Vestibular hypofunction which currently affects an estimated 403 million to 725 million people worldwide, representing 5% to 9% of the global population. In parallel, more than 285 million suffer from severe visual impairment. The economic and societal burden is immense, underscoring a critical need for transformative scientific and medical innovation.
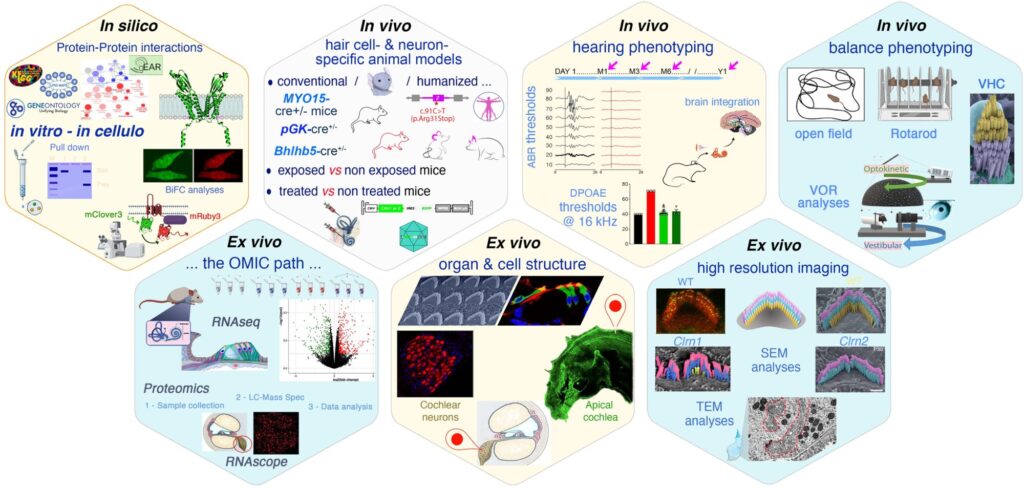
We combine cutting-edge approaches across multiple disciplines—genetics, molecular biology, advanced imaging, electrophysiology, and behavior—to investigate the molecular, cellular, and systems-level mechanisms of hearing, balance, and vision deficits. Our multiscale and integrative phenotyping pipeline enables deep functional and structural profiling of disease models. This allows us to map disease trajectories with exceptional precision and to assess early-stage disruptions that precede irreversible damage.
At the Progressive Sensory Disorders (PSD), a research unit at the Institut de l’Audition, Institut reConnect, center of the Institut Pasteur, we are committed to advancing the frontiers of sensory neuroscience and therapy. Our research addresses the complexity of disorders affecting the inner ear and retina, with a special interest to progressive conditions that often begin postnatally and worsen over time.
Our work centers on Usher syndrome, a model for progressive deaf-blindness, and expands to other causes of late-onset sensory loss, including age-related and noise-induced hearing impairments. By leveraging both murine and porcine models, we perform cross-species analyses that mirror clinical diversity in symptom onset, severity, and progression.
Three Interconnected Research Aims
We approach sensory disorders through three primary research objectives:
- Deciphering Disease Mechanisms—From molecular signatures to physiological and behavioral outcomes, we aim to identify the biomarkers and pathways underlying progressive sensory dysfunction. These insights support early diagnosis and targeted interventions.
- Investigating External Modulators—We study how aging, environmental noise, inflammation, and oxidative stress influence disease onset and progression, identifying modifiable factors that shape patient outcomes.
- Developing Therapeutic Solutions—Using gene supplementation, mini-genes, dual-AAV systems, and gene editing technologies (e.g., CRISPR), we design and test novel treatments. Our goal is to develop scalable, mutation-specific therapies that restore sensory function and slow or prevent degeneration.
Our cross-disciplinary strategy enables parallel evaluation of therapies and biomarkers in diverse models and conditions—crucial for personalizing medicine and ensuring translational success.
Our team includes principal investigators, engineers, postdoctoral fellows, and students who are passionate about linking science with therapeutic innovation. We maintain an active interface with the broader scientific, clinical, and public communities.
We welcome inquiries from motivated researchers, academic and industry collaborators.
Contact: aziz.el-amraoui@pasteur.fr | PSD-PI@pasteur.fr
Below are key recent research highlights illustrating our discoveries in gene function, disease mechanisms, and therapeutic innovation for progressive hearing loss and Usher syndrome, with direct implications for diagnosis, model development, and treatment strategies.
Highlight 1: From Gene Discovery to Therapeutic Innovation in Progressive Hearing Loss
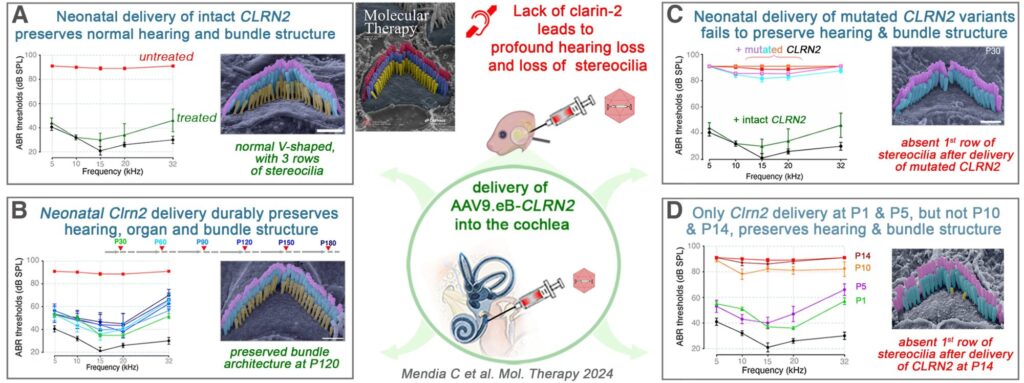
Our team, in collaboration with Mike Bowl (UCL ear Instiutute) and Barbara Vona (InnerEarLab Gottingen), identified and characterized CLRN2 (Clarin-2) as a critical gene for auditory function, demonstrating its role in maintaining stereocilia integrity and hair cell synaptic function. Loss-of-function mutations in CLRN2 lead to progressive hearing loss in mice and humans, establishing CLRN2 as a novel deafness gene (DFNB117) (EMBO Mol. Med., 2019; Hum. Genet., 2021).
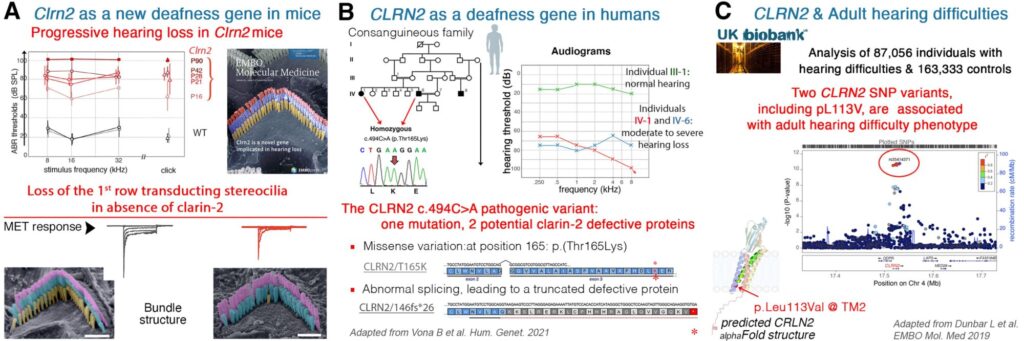
We developed a gene therapy strategy using AAV9-PHP.eB vectors to deliver Clrn2 into neonatal Clrn2-deficient mice. This approach durably preserved hearing across all frequencies by maintaining stereocilia structure and synaptic function up to one year post-injection (Mol. Ther., 2024). Importantly, we showed that therapeutic efficacy declines significantly when delivery occurs after postnatal day 5, pinpointing a narrow therapeutic window.
Highlight 2: Rethinking Sensory Syndromes—Clarifying CIB2 and Usher Vision Loss
We redefined the role of CIB2 in hereditary hearing loss, disqualifying its previously assumed involvement in Usher syndrome. Our studies showed that CIB2 mutations cause non-syndromic hearing loss, with no vestibular or visual defects typical of Usher type 1. This finding, supported by both genetic and animal model data (EMBO Mol. Med., 2017; Clin. Genet., 2018), reshaped diagnostic criteria and improved genetic counseling protocols (Hum. Genet., 2022).
Highlight 3: Evolutionary Adaptation and Molecular Innovation in Sensory Systems
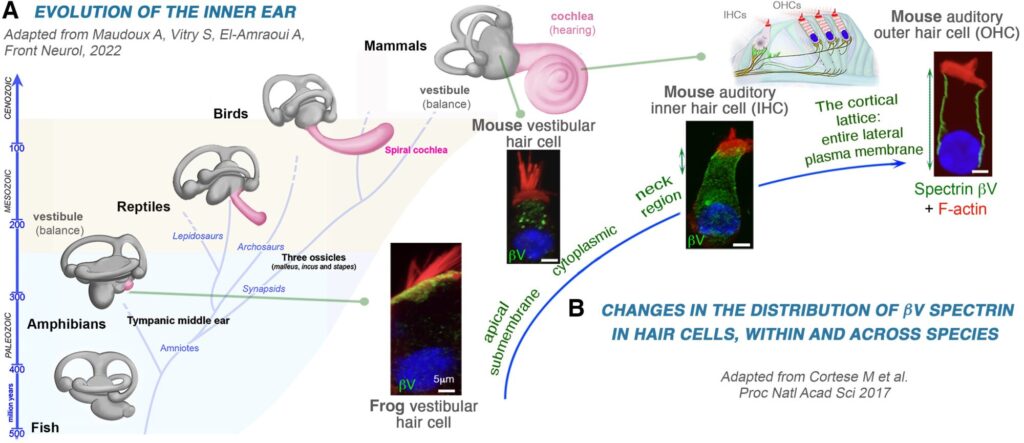
Our investigations into the evolutionary biology of sensory organs revealed how specific molecular innovations, such as those involving spectrin βV, contributed to the functional sophistication of hearing and vision in vertebrates. Spectrin βV, an unusually large cytoskeletal protein, was shown to have undergone mammal-specific positive selection (PNAS, 2017), driving its redistribution from the apical surface (in amphibians) to lateral membranes in mammalian outer hair cells—cells responsible for cochlear amplification.
We also discovered spectrin βV’s role in retinal photoreceptors, where it interacts with Usher proteins and molecular motors to support intracellular trafficking (Hum. Mol. Genet., 2013). These findings illustrate how subtle evolutionary shifts in protein localization and function can result in key sensory evolved specialisations.
Simultaneously, our research resolved a longstanding puzzle in Usher syndrome: why do human patients suffer from retinal degeneration while mouse models do not?
Highlight 4: Solving the Usher Vision Loss Paradox—The Role of Calyceal Processes
Why do Usher type 1 patients lose vision, while mouse models do not? We resolved this long-standing paradox by identifying calyceal processes—actin-based structures in human photoreceptors—as essential targets of USH1 proteins (J. Cell Biol., 2012). We demonstrated that calyceal processes—actin-rich structures absent in mice but present in humans and other species—are crucial for photoreceptor maintenance and are severely disrupted in Usher syndrome (J. Cell Biol., 2017). This discovery redefined the field, highlighting the need for alternative models to study Usher retinal disease and guiding new therapeutic strategies (cf. Hum. Genet., 2022).
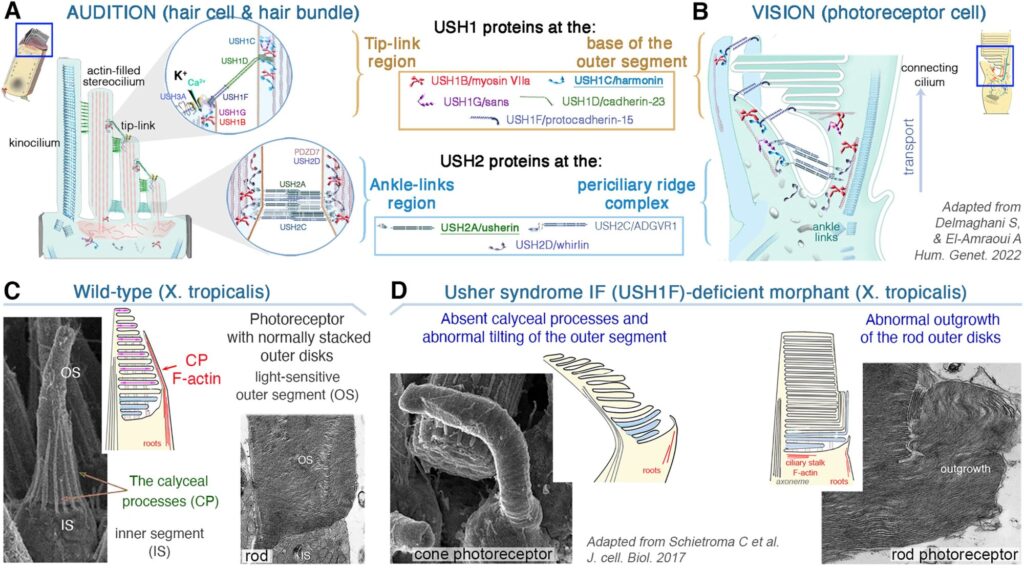
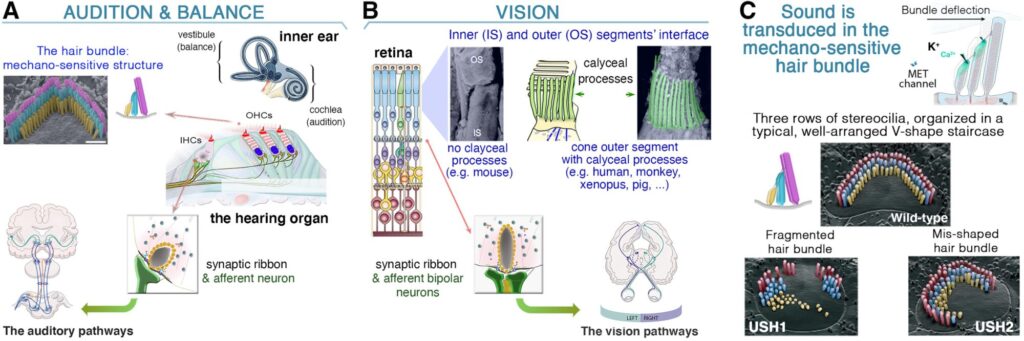
(A-B) Usher proteins form molecular networks in photoreceptor cells and hair cells, linking cytoskeletal and membrane structures. The Usher proteins-mediated network take place at the inner (IS) and outer (OS) segment interface of the photoreceptor cells (A), and at the hair cells’ hair bundle (B). The two regions display ciliary (microtubule)-based structures combined with highly specialized membrane/microvilli-like (F-actin) structures (reviewed in Hum Genet 2022). (C-D) Defects in calyceal processes disrupt outer segment morphology, causing Usher-related retinopathy.
Job opportunity

























