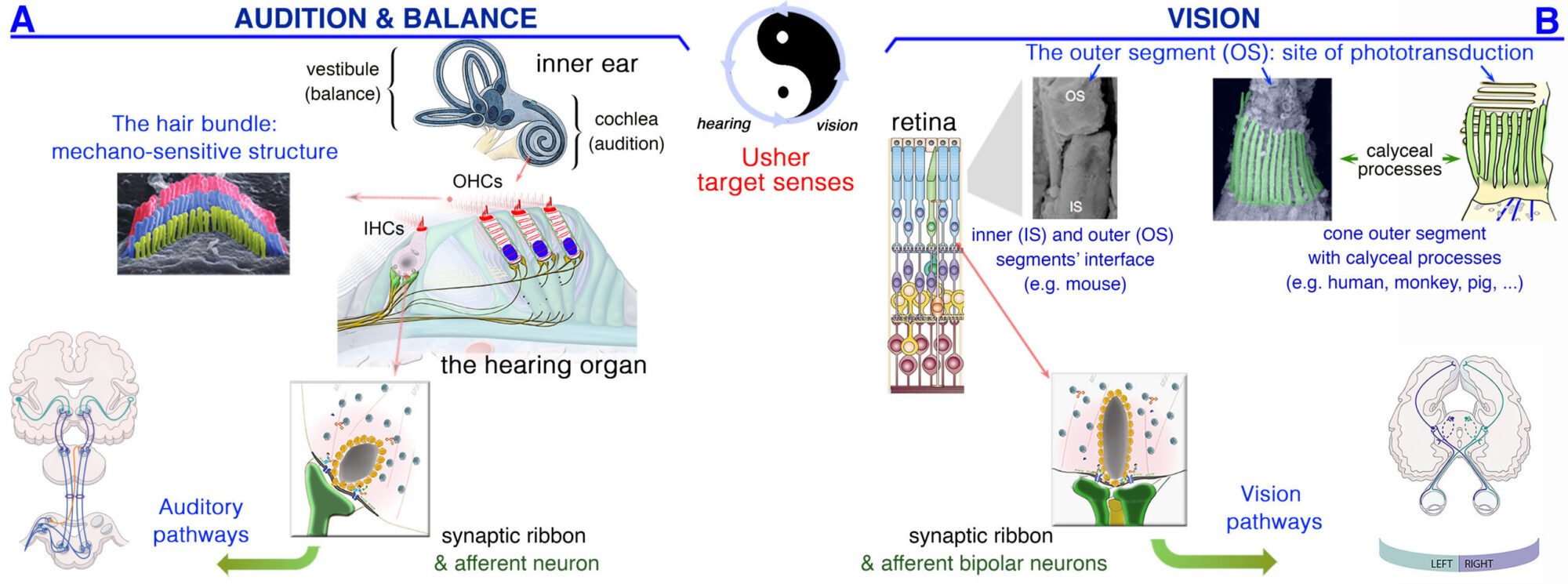
The study of the inner ear and retinal sensory systems has become, in many cases, a blueprint for aspects pertinent to the nervous system and related neurodegenerative disorders. The senses of vision, balance, and hearing (Fig. 1) are essential for every significant activity of daily life, ranging from social interactions and mobility to an appreciation of music, art, and nature. Consequently, impairment of these sensory modalities has a profound negative impact in the quality of life of the affected individuals, which range from communication constraints and lower access to entertainment and working opportunities. In turn, this can lead to social isolation and depression 1-5. From comprehensive empirical data, the World Health Organization estimates that around 285 million people currently display severe vision impairments worldwide (see http://www.who.int/). Also, 460 million people — representing 5% of the world’s population — have a disabling hearing impairment (with or without balance deficit). This number is expected to increase to more than one billion people by 2050 (see http://www.who.int/). Altogether, these sensory deficits have a dramatic economic impact on healthcare systems and society as a whole.
Over the years, we showed that, even though processing different sensory signals — mechanical stimulation in hair cells and light inputs in photoreceptors—, the inner ear and retinal sensory cells both display membrane- and cytoskeleton-based structures that are key to hearing, balance and vision functioning (Fig. 1). The research interests of our lab span the inner ear and the retinal sensory systems, from animal model to human patients, from normal to abnormal function, from organ architecture to behavior, and from the molecular and genetic bases of hearing, balance, and/or vision deficits to innovative treatments solutions.
In the Progressive Sensory Disorders team, our targets are the postnatal, progressive hearing (± balance/vision) disorders. In many cases, progressivity and severity differ among patients, but the origin and the mechanisms involved remain elusive and treatment of such deficits are still missing. We believe that a gene-environment interplay probably determines individuals’ vulnerability and degree of sensory decline. Using total-, hair cell-, and/or neuron-specific inactivation of selected causal deafness genes (sometimes associated with balance and vision loss, e.g. CLRN1 & CLRN2), we reproduce patients’ various clinical conditions in animal models that we use as model systems to elucidate precise disease mechanisms, and identify disease risk-conferring pathways. Our established multiscale interdisciplinary and integrative approach include computational, “OMICs”, biochemical, high-resolution imaging, physiological and behavioral experiments put together to unveil key pathways in health and disease, under standard or “challenged” (e.g. noise-exposure, aging) conditions.
By collecting and integrating information from whole organisms, through intracellular events (e.g., hair bundle, synapse), down to key structural and functional properties of the defective proteins, our findings make it possible to:
i) Decipher key pathogenic pathways involved in progressive hearing, balance, and/or vision impairments,
ii) Depending on gene of interest, unlock the subtle clinical/phenotypic changes among patients, which help identify potential protective pathways, and single out risk factors prone to sensory decline,
iii) Through the lens of a specific gene, seek the potential impact on hearing of gene-environment interactions, & how such interactions vary overtime with ageing.
iv) As the late-onset and/or progressive hearing deterioration offer the possibility to intervene long-enough before possible irreversible damage, we also design gene therapeutics (gene replacement, gene editing) to weigh their efficacy to prevent, slow down and/or correct sensory decline.
Our projects call for multidisciplinary and integrative approaches, taking advantage of multiple internal, national and international collaborations. The lab is staffed by a dynamic team, with 5 permanent staff members: the PI, Aziz El-AMRAOUI, two Assistant Professors: Sedigheh DELMAGHANI (strong expertise in genetics, molecular biology and cell biology of the hearing organ, including experience with oxidative stress and noise-induced hearing loss; Sandrine VITRY (strong expertise in molecular physiopathology and physiology of neurodegenerative disorders, and therapeutic neuroprotection strategies), and two technicians: Sylvie NOUAILLE & Sebastien LE GAL, with complementary expertise, ranging from animal handling and management to biochemistry, molecular and cell biology of the inner ear. Audrey MOUDEAUX is an ENT physician (Expert in vestibulopathies, working halftime in the lab).
Five temporary positions are now part of the team: Three POST-DOCs: Emilia WYSOCKA (POL), Muge SENARISOY (TUR), Samantha PAPAL (FRA), two PhDs: Maureen WENTLING (USA), Clara MENDIA (ARG). Also, we host on average 2 to 4 internships per year.
If you want to join the team or have questions about our work, please feel free to contact us
(aziz.el-amraoui@pasteur.fr or PSD-PI@pasteur.fr).
Thank you for visiting our website
In absence of a cure, untreated decline of vision, balance and/or hearing have huge economic & societal impact worldwide. Whatever the cause – i.e., genetic, environment (noise, ototoxic drugs, light-phototoxicity, …), or aging – loss of hearing, balance, and/or vision is often linked to irreversible loss of the specialized sensory cells in the inner ear (hair cells) and the eye (photoreceptors), and associated neurons. We study disorders targeting these sensory cells as model systems to tackle neurodegenerative disorders from disease mechanisms to therapeutic options.
Figure 1: Similarities between the sensory organs of vision, hearing & balance. (A) Hearing is dependent on the processing of sound waves within the hair bundle, which crown the inner (IHCs) and outer (OHCs) auditory hair cells in the cochlea. (B) Light signals are transduced by the outer segments of the photoreceptors, in the retina. In the hair cells (A) and photoreceptors (B), the neurotransmitter (Glutamate) is released in synaptic active zones different from those of brain conventional synapses in that they are associated with an electron-dense ribbon surrounded by tethered synaptic vesicles.
Summaries of some last achievements (2015-2020) : https://research.pasteur.fr/wp-content/uploads/2015/07/research_pasteur-enprogressive-sensory-disorders-pathophysiology-and-therapyfrdeficits-sensoriels-progressifs-pathophysiologie-et-therapie-psd-lab-achievements-2015-2020.pdf