Genetic and epigenetic mechanisms controlling immune cell functions
Immune-mediated inflammatory diseases such as rheumatoid arthritis, spondyloarthritis (SpA), inflammatory bowel disease and psoriasis cause significant morbidity and are a substantial burden for the affected individuals and the society. Our very limited understanding of the pathogenic mechanisms currently hampers the development of better markers for early diagnosis and more specific and effective therapies.
The main goal of the Immunoregulation Unit is to unravel the fundamental mechanisms that control the differentiation and function of human T lymphocyte populations with pro-inflammatory and regulatory activities and to study their roles in the pathogenesis of inflammatory diseases.
During the past years, we have performed an integrated analysis of the signaling pathways, epigenetic modifications and genetic networks involved in T helper cell differentiation. We have studied how signals originating at the T cell receptor (TCR) and at cytokine receptors are integrated to shape a T helper subset-specific gene expression programs in primary human T lymphocytes. We have analyzed in particular the synergy of transcription factors and chromatin remodelling complexes to induce epigenetic changes and gene transcription during T helper type 1 (Th1) cell development.
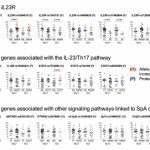
More recent work focuses on the impact of genetic variation on CD4+ T cell functions in chronic inflammatory diseases and analyzes the role of subpopulations of human regulatory T cells in patients after allogeneic hematopoietic stem cell transplantation. The lab also studies epigenetic mechanisms that control the stability and plasticity of CD4+ T cell subsets.