Link to Pubmed [PMID] – 15075282
Eukaryotic Cell 2004 Apr;3(2):536-45
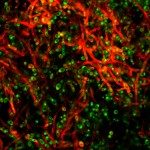
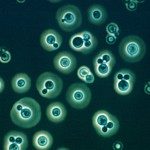
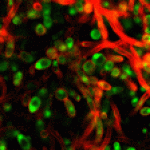
Like many bacteria, yeast species can form biofilms on several surfaces. Candida albicans colonizes the surfaces of catheters, prostheses, and epithelia, forming biofilms that are extremely resistant to antifungal drugs. We have used transcript profiling to investigate the specific properties of C. albicans biofilms. Biofilm and planktonic cultures produced under different conditions of nutrient flow, aerobiosis, or glucose concentration were compared by overall gene expression correlation. Correlation was much higher between biofilms than planktonic populations irrespective of the growth conditions, indicating that biofilm populations formed in different environments display very similar and specific transcript profiles. A first cluster of 325 differentially expressed genes was identified. In agreement with the overrepresentation of amino acid biosynthesis genes in this cluster, Gcn4p, a regulator of amino acid metabolism, was shown to be required for normal biofilm growth. To identify biofilm-related genes that are independent of mycelial development, we studied the transcriptome of biofilms produced by a wild-type, hypha-producing strain and a cph1/cph1 efg1/efg1 strain defective for hypha production. This analysis identified a cluster of 317 genes expressed independently of hypha formation, whereas 86 genes were dependent on mycelial development. Both sets revealed the activation of the sulfur-amino acid biosynthesis pathway as a feature of C. albicans biofilms.