Link to Pubmed [PMID] – 26165863
Link to DOI – 10.1016/j.ajpath.2015.04.027S0002-9440(15)00327-2
Am J Pathol 2015 Sep; 185(9): 2421-30
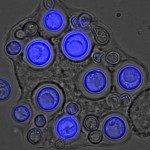
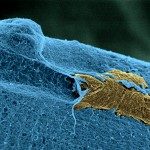
Clinical data and experimental studies suggest that bronchial epithelium could serve as a portal of entry for invasive fungal infections. We therefore analyzed the interactions between molds and the bronchial/bronchiolar epithelium at the early steps after inhalation. We developed invasive aspergillosis (Aspergillus fumigatus) and mucormycosis (Lichtheimia corymbifera) murine models that mimic the main clinical risk factors for these infections. Histopathology studies were completed with a specific computer-assisted morphometric method to quantify bronchial and alveolar spores and with transmission electron microscopy. Morphometric analysis revealed a higher number of bronchial/bronchiolar spores for A. fumigatus than L. corymbifera. The bronchial/bronchiolar spores decreased between 1 and 18 hours after inoculation for both fungi, except in corticosteroid-treated mice infected with A. fumigatus, suggesting an effect of cortisone on bronchial spore clearance. No increase in the number of spores of any species was observed over time at the basal pole of the epithelium, suggesting the lack of transepithelial crossing. Transmission electron microscopy did not show spore internalization by bronchial epithelial cells. Instead, spores were phagocytized by mononuclear cells on the apical pole of epithelial cells. Early epithelial internalization of fungal spores in vivo cannot explain the bronchial/bronchiolar epithelium invasion observed in some invasive mold infections. The bioimaging approach provides a useful means to accurately enumerate and localize the fungal spores in the pulmonary tissues.