About
The Spectral Cytometry technology at the Cytometry Platform, CB UTechS
A vision for the future of cytometry
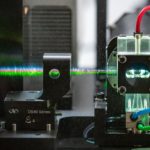
High parameter cell analysis with the capability to analyze tens of thousands of cells enables biological and medical research in many areas like immunology and microbiology. To achieve high analysis rates and to overcome limits of manual microscopy, flow cytometry was developed several decades ago. Rapid technology progress in light sources, detectors, high speed analog and digital electronics combined with the development of new dyes, affinity reagents and algorithms provided new capabilities for flow cytometry. The ability to measure multiple fluorescent spectra makes a spectral flow cytometer more flexible than a conventional flow cytometer. Our new equipment has capabilities that exceed conventional flow cytometry methods (6 lasers, 188 detectors). Spectral detection is determined with light dispersion elements such as prisms and diffraction gratings.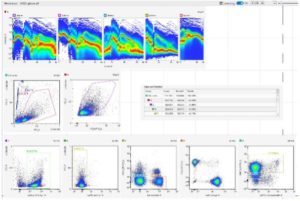
The major advantage of spectral flow cytometry is the ability to measure the whole fluorescence spectrum for each individual fluorophore in the sample. Additionally, cellular autofluorescence can be used as a parameter, which is an additional powerful approach for the characterization of specific cells tissues. Our platform is on of the advanced users of the spectral technology, our first publication demonstrating the fantastic capacity of spectral flow has been publish in 2016 and it is often cited as a reference paper
References :
- Ferrer-Font L et al. (2020). Panel design and optimization for high‐dimensional immunophenotyping assays using spectral flow cytometry. Curr Protoc in Cytom 92, e70.
- Robinson JP (2019). Spectral flow cytometry — Quo vadimus? Cytometry Part A 95, 823-824.
- Schmutz S (2016). Spectral Cytometry has a unique property allowing multicolor analysis of cell suspensions isolated from solid tissues, PLOS ONE, August 2016
- Nolan JP and Condello D (2013). Spectral flow cytometry. Curr Protoc Cytom Chapter: Unit 1, 27.
Enhanced optics
Conventional flow cytometry relies on bandpass filters and dichroic mirrors to isolate a narrow wavelength band around the emission peak of each fluorophore so that each detector is dedicated to collecting photons emitted from one color. Spectral flow cytometers, instead, use dispersive optics, such as prisms or gratings, that scatter photons according to wavelength across an array of detectors. By capturing the entire visible and near-infrared spectrum of each fluorophore, spectral flow cytometry can distinguish fluorophores with very similar emission wavelengths if they have distinct signatures across the spectrum.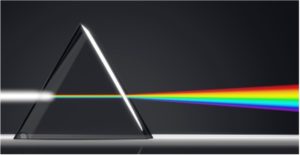
Improved detectors
Conventional flow cytometry uses photomultiplier tubes (PMTs) to measure fluorescence in specific wavelength bands. PMTs have lower quantum efficiencies in the red and far-red regions, thus effectively limiting the use of fluorescent molecules that emit in these regions. When designing large multicolor panels, it becomes imperative to be able to include these dyes in the analysis. Furthermore, PMTs increase the footprint and the cost of a cytometer. The detectors of choice for spectral flow cytometry are multianode PMTs or charged coupled devices. PMTs have lower quantum efficiency and resolution than CCDs, especially in the red regions, but have much higher gain and faster speed. The increased efficiency and the smaller size of the newer detectors make it possible to build an array of detectors that can capture the entire spectrum of the fluorophore, increasing sensitivity and resolution.
Spectral analysis and spectral unmixing
The deconvolution of the spectral profile to distinguish the contribution of the different fluorophores is known as spectral unmixing. One of the most used unmixing algorithms is the classical least squares method. It is a supervised approach, used when the spectrum of the individual components is known. The Multivariate Curve Resolution algorithm is instead an unsupervised method, used when nothing is known about the individual spectral components.
References :
- Sahir F, Mateo JM, Steinhoff M, Siveen KS. Development of a 43 color panel for the characterization of conventional and unconventional T-cell subsets, B cells, NK cells, monocytes, dendritic cells, and innate lymphoid cells using spectral flow cytometry. Cytometry A. 2020 https://doi.org/10.1002/cyto.a.24288
- Robinson, J. P. (2019). Spectral flow cytometry- Quo vadimus? Cytom. A. 95 (8), 823–824. doi:10.1002/cyto.a.23779
- Nolan JP, Condello D. Spectral flow cytometry. Curr Protoc Cytom. 2013;Chapter 1:doi:10.1002/0471142956.cy0127s63 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3556726/
- John P. Nolan, Danilo Condello, Erika Duggan, Mark Naivar, David Novo Visible and near-infrared fluorescence spectral flow cytometry J, 2012 https://doi.org/10.1002/cyto.a.22241