About
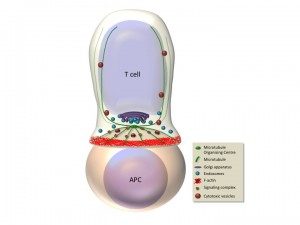
Antigen recognition by the T cell receptor triggers extensive rearrangements of both the actin and microtubule cytoskeleton. Cytoskeleton changes condition in turn morphological changes and molecular reorganization at the immunological synapse (Figure). We have investigated the molecules that regulate cytoskeleton reorganization in response to T cell receptor signals. In particular, we investigated ERM (ezrin, radixin, moesin) proteins, since they link membrane components with the actin cytoskeleton (Charrin and Alcover, 2006; Lasserre et al. 2010; Soares et al, 2013), and polarity regulators, like Dlg1 and Adenomatous polyposis coli (Apc), since they regulate cytoskeleton reorganization in polarized cells. We have shown that ezrin relocalizes to the periphery of the synapse, it interacts with Dlg1 and together control the interplay between filamentous actin and microtubules, ensuring microtubule network organization at the immunological synapse. In this way, these proteins regulate the dynamics of signaling complexes at the synapse and as a consequence T cell receptor signal transduction. Moreover, these proteins control T cell activation leading to cytokine production by regulating the activation of the transcription factor NFAT (Roumier et al. 2001; Das et al., 2002; Lasserre et al 2010). We have recently shown that another polarity regulator named Adenomatous polyposis coli (Apc), which interacts with Dlg1, is also involved. Apc is a tumor supressor whose mutations are associated with familial adenomatous polyposis and colorectal cancer development. Although extensively studied in epithelial transformation, the role of Apc in T lymphocyte physiology was poorly known. We have shown that Apc is necessary for microtubule network organization at the immunological synapse, and for T cell receptor-triggered activation through the NFAT transciption factor. Indeed, Apc is necessary for NFAT’s nuclear localization and for NFAT-driven transcription leading to cytokine gene expression. Interestingly, NFAT forms microclusters juxtaposed with microtubules and needs microtubules to efficiently localized in the nucleus upon T cell activation. Finally, mouse Apc deficiency reduces the presence of NFAT in the nucleus of intestinal regulatory T cells (Tregs) and impairs Treg differentiation and the acquisition of a suppressive phenotype characterized by the production of the anti-inflammatory cytokine IL-10 (Agüera-Gonzalez et al. 2017). These findings suggest a dual role for Apc mutations in colorectal cancer development, where mutations drive the initiation of epithelial neoplasms and also reduce Treg-mediated suppression of the detrimental inflammation that enhances cancer growth
Moreover, we have unveiled an interesting interplay between endosomal traffic and actin cytoskeleton remodeling in T cells. Thus, we have shown that actin cytoskeleton remodeling and, as a consequence, T cell shape and immunological synapse symmetry are influenced by the endosomal traffic of a key actin cytoskeleton regulator, the small GTPase Rac1. Indeed, we observed Rac1 in a type of recycling endosomes characterized by the presence of another small GTPase, Rab11. Rac1 transport by recycling endosomes is modulated by the interplay between Rac1 and Rab11, which occurs through a Rab11-interacting protein called FIP3. Therefore, endosomal traffic and actin cytoskeleton remodeling are orchestrated processes that cooperate to ensure the structure and function of immunological synapses (Bouchet et al. 2016). Finally, we have shown that the human immunodeficiency virus HIV-1 subverts, through its accessory protein Nef, Rac1 endosomal traffic, modulating in infected T cells actin cytoskeleton reorganization during T cell spreading (del Rio-Iñiguez et al. 2018).