About
Our team identified the first mutations in the SHANK3 gene, showing it’s role in autism and Phelan-McDermid Syndrome (PMS). Thanks to a donation from the French Association of Phelan-McDermid Syndrome, we will continue the whole genome sequencing of PMS patients in order to identify genomic regions associated with the severity of clinical symptoms. This study will lead to better diagnosis and to the identification of new therapeutic targets.
Phelan-McDermid syndrome (PMS) is a neurodevelopmental disorder with non-specific clinical features. The main symptoms are hypotonia, intellectual disability (ID), absent or delayed speech together with minor dysmorphic features. More than half of the patients exhibit autism spectrum disorders (ASD).
PMS is a contiguous gene syndrome resulting from deletion of the distal long arm of chromosome 22. The type and the size of the deletion can vary from one patient to another. In the vast majority of the cases, the deletion is de novo.
There is a gradient of severity in PMS. Some patients have difficulty to walk or may have a severe intellectual disability while other patients can speak and go to high school. The size of the deletion seems to contribute to the presence of some features (speech problems are more frequent in patients with large deletions). In contrast, seizures, hypotonia, birth weight, and gestational age at birth do not seem to correlate with deletion size.
Among the genes located at 22q13, we previously identified deleterious de novo mutations of the SHANK3 gene in patients with ASD and ID. SHANK3 codes for a synaptic scaffolding protein that plays an important role in the formation of dendrites and glutamatergic synapses. While SHANK3 has been extensively studied as a candidate gene for the neurological symptoms of PMS, it is clear that many other genes in the region are involved in the pathogenesis of PMS.
Projects
Genetic and phenotypic diversity of patients with PMS in Europe
In order to identify factors that influence the severity of the syndrome, we aim to analyze the relationship between genotype and phenotype in patients with PMS. Instead of focusing only on the 22q13 region, we want to analyze the whole genome of each patient in order to identify genetic variations that will contribute to the severity of the disorders. For example, we want to identify which genes will contribute to the risk of developing epilepsy.
Cellular and mouse models of PMS
In order to identify the mechanisms underlying the syndrome and the main neural circuits involved, we are currently studying cellular and mouse models of PMS. Our cellular models consist of neurons derived from induced pluripotent stem cells (iPSC) from PMS patients with autistic disorders and carrying a de novo mutation altering the function of one copy of the SHANK3 gene. Our mouse models are mice that have lost part or all of the Shank3 gene.
TO COME: Effect of lithium in patients with both autism spectrum disorder and Phelan-McDermid syndrome (SHANK3 haploinsufficiency), phase 2 pilot study
To date, there is no pharmacological treatment that has shown activity on the symptoms of these disorders, in particular social communication deficit. However, we have previously shown that lithium can restore synaptic dysfunction caused by a defect in the SHANK3 gene. The primary objective of the current clinical trial is to evaluate the effect of lithium at 12 weeks (capsules dosed at 62.5mg, 125mg and 250mg), compared to placebo, on social communication deficit in patients with Phelan-McDermid syndrome.
Below are the patient inclusion criteria for the lithium trial:
- Children aged 7 years to 17 years and 8 months
- Minimum weight of 19 kg for children aged 7 years
- Patient with SHANK3 haploinsufficiency, i.e., carrier of a deletion (CNV) involving the SHANK3 gene or a de novo truncating mutation in SHANK3 (Phelan-McDermid syndrome)
- Autism spectrum disorder
- Sexually active patients of childbearing potential must agree to use a highly effective form of contraception during the study period (estrogen/progestin contraception, or intrauterine device, or abstinence)
- Affiliation to social security system
- Signature of consent by the holders of parental authority
- Non-participation in another clinical trial at the same time
Constraints related to participation in the study: recurrent blood sampling during the first month (once a week) at the Robert-Debré Hospital in Paris.
Our collaborators
Foundations and collaborative groups
- French Association of the Phelan-McDermid syndrome
- Phelan-McDermid Syndrome Foundation, USA
- Phelan-McDermid Syndrome Foundation, UK
- Italian Association of the Phelan-McDermid syndrome
- European consortium EU-AIMS
- Association Téhani et les enfants Phelan-McDermid, France
Clinicians/Geneticists
- USA (New Orleans): Dr Katy Phelan, Tulane University
- France (Paris): Pr Richard Delorme, Dr Anna Maruani, Centre d’excellence pour l’autisme et les trouble du neurodéveloppement (InovAND), CHU Hôpital Robert-Debré
- France (Paris): Pr Anne Claude Tabet, Service de cytogénétique, CHU Hôpital Robert-Debré
- France (Paris): Pr Thierry Bienvenu, Service de génétique moléculaire, CHU Paris Centre, Hôpital Cochin
- France (Nîmes): Pr Serge Lumbroso, CHU of Nîmes
- France (Angers): Pr Dominique Bonneau, CHU of Angers
- France (Clermont-Ferrand): Dr Christine Francannet, CHU of Clermont-Ferrand
- Belgium (Louvain): Pr Hilde Van Esch, University of Louvain
- Denmark (Odense): Dr Christina Fagerberg, Hôpital universitaire of Odense
- Netherlands (Groningen): Pr Conny van Ravenswaaij-Arts: 22q13@umcg.nl
- Germany (Ulm): Dr Sarah Jesse: sarah.jesse@uni-ulm.de & Michael Schoen: michael.schoen@uni-ulm.de, Universitäts- und Rehabilitationskliniken Ulm
- UK (London): Eva Loth: eva.loth@kcl.ac.uk, Institute of Psychiatry, King’s College
- Italy (Milan): Stefano D’Arrigo, Istituto Neurologico Carlo Besta: Arrigo@istituto-besta.it
- Italy (Pisa): Filippo Muratori & Filippo Santorelli: filippo.muratori@fsm.unipi.it, filippo.santorelli@fsm.unipi.it, Istituto Stella Maris
- Italy (Rome): Pr Marcella Zollino: marcella.zollino@unicatt.it, Università Cattolica del Sacro Cuore
- Sicilia (Troina): Dr Maurizio Elia: melia@oasi.en.it, Ospedale Oasi di Troina
Neurobiologists
- Germany (Ulm): Pr Tobias Boeckers: tobias.boeckers@uni-ulm.de, University of Ulm
- Italy (Milan): Dr Carlo Sala, University of Milan
- Italy (Milan): Dr Chiara Verpelli, University of Milan
- France (Évry): Dr Alexandra Benchoua, Institut des cellules souches pour le traitement et l’étude des maladies monogéniques (I-Stem)
- France (Paris): Dr David DiGregorio, Pasteur Institute
- France (Paris): Dr Laure Rondi-Reig, Institute of Biology Paris-Seine
Please visit the Frontiers Research Topics on Shankopathies.
About SHANK2 disorders? Check the website of the recent SHANK2 Foundation! Register your child in the SHANK2 Patient Registry and help it grows.
Our recent papers on the Phelan-McDermid syndrome and SHANK3:
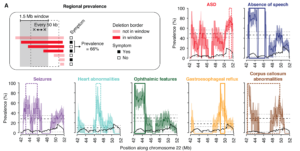
A framework to identify contributing genes in patients with Phelan-McDermid syndrome
Anne-Claude Tabet, Thomas Rolland et al.
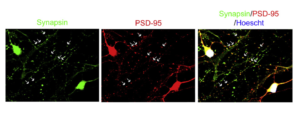 Human pluripotent stem cell-derived cortical neurons for high-throughput medication screening in autism: a proof of concept study in SHANK3 haploinsufficiency syndrome
Human pluripotent stem cell-derived cortical neurons for high-throughput medication screening in autism: a proof of concept study in SHANK3 haploinsufficiency syndrome