“Science is not about accumulating new data: it is the ability to change the way we think about data, is the ability to break prejudices and to ask the next (right and simple) question”
Chiara Zurzolo, MD, PhD, received her doctorate degrees from the University of Naples “Federico II” before spending 4 years as a postdoctoral fellow at Cornell Medical School in New York. She returned to Naples University Medical School to establish her own research group as an Assistant and then Associate Professor. In 2003, she moved to Paris as a Director of Research. In 2015 she was nominated Professor at the Pasteur Institute and elected as an EMBO Member.
Zurzolo is an internationally well-known researcher, very often invited to speak at meetings around the globe. She is also highly involved in teaching at Pasteur and internationally, organizing and participating in high-level courses for Master and PhD students. She has also been active in other organizations such as ELSO, demonstrating her energy and engagement for promoting science.
Chiara Zurzolo has made several seminal discoveries in the fields of cell biology and neurodegeneration. During her postdoc, she focused on apical sorting mechanisms of GPI-anchored proteins and showed (against the prevailing hypothesis) that the GPI-anchor is NOT an apical signal. Her recent findings suggest that the sorting mechanism of GPI-APs in the Golgi regulates their apical organization and function (Nat Chem Biol 2014). This regulatory sorting mechanism is a novel concept that opens new avenues to elucidate conditions in which polarity is lost, such as in cancer, the current focus of several projects in her lab.
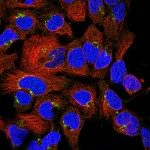
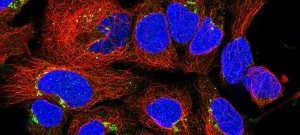
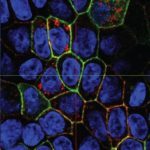
She started working on prion trafficking at the Pasteur Institute in 2003. She uncovered the intracellular site of pathological conversion of the prion protein (PlosPath 2009) and the mechanism of prion dissemination to the brain. She showed that prion dissemination between neurons and from dendritic cells to neurons occurs by Tunneling Nanotubes (TNTs), a new mechanism of direct intercellular communication (Nat Cell Biol 2009). Importantly, she has found that this mechanism is relevant for the intercellular spreading of other amyloid aggregates involved in neurodegenerative diseases (JCS 2013, BJ review 2013, Sci Rep 2015, EMBO J 2016, Biol Open 2017, ActaNeu 2017). She proposes that TNTs are involved in the spreading of different amyloidogenic proteins in the brain (JCB 2017) and therefore represent a suitable target to halt incurable neurodegenerative diseases. This represents a new paradigm in neurodegenerative diseases.
Her laboratory is now working on further assessing the role of TNTs in neurodegenerative diseases as well as in different cancer models, and to understand the molecular mechanisms involved in TNT formation. Her group discovered that TNTs and filopodia are molecularly and structurally different (Sci Rep 2016, JCS 2018, Nat Comm, under revision), representing novel organelles. Currently, she and her group are concentrating on identifying these structures in vivo, normal and diseased conditions. Her fundamental cell biology work has excellent translational potential in many fields where TNTs might be involved (e.g. cancer, development, immunity, etc.).