Présentation
Human Papillomaviruses (HPV) are the major cause of human cervical cancer. We have developed a therapeutic tumor vaccine, the CyaA-E7 vaccine, that can induce specific responses against the HPV E7 oncoprotein and eliminate E7 expressing tumors, such as TC-1. This vaccine is currently under Phase II clinical trial (http://www.genticel.com/). Tumor immunosuppression represents the major cause of the failure of many cancer therapies. Most of the immunotherapies found efficient in mice are indeed inefficient in cancer patients, indicating that traditional mouse tumor models do not recapitulate the human situation. We thus developed a “failure” model in which we delayed the treatment of mice-bearing TC-1 tumor by the CyaA-E7 therapeutic vaccine and demonstrated that the therapeutic efficacy of the vaccine is progressively lost as the tumor grows. To understand and overcome these suppressive responses, we are analyzing the interaction between tumor cells and the immune system during tumor growth. Our analysis shows that along with the increase of the tumor volume, several immunosuppressive cells accumulate in TC-1 tissue, especially Foxp3+ regulator T cells (Treg cells) and myeloid derived suppressor cells (MDSC). The accumulation of these cells may explain the decrease of vaccine’s efficiency.
Cellular infiltrates within the tumor microenvironment
The accumulation of CD4+ Tregs in tumor tissue is a widely described phenomenon in mouse models and in human cancer patients. Understanding the mechanisms by which Treg cells migrate and accumulate into tumors is of primary importance for the development of successful immunotherapies. We used the immunoscope technology to analyze the TCR repertoire of tumor-infiltrating T cells. We showed that tumor-infiltrating Treg and effector T cells (Teff cells) displayed CDR3 spectratyping profiles characteristic of biased and strongly perturbed repertoires, typical of clonal expansions, suggesting that strong T-cell responses have occurred within the tumor tissue. Comparison of the TCR sequences of tumor-infiltrating Tregs demonstrated an extremely high percentage of overlapping sequences inside a BV family revealing the existence of public sequences. These public TCR sequences were tumor specific and were not shared between Treg and Teff tumor-infiltrating T-cells, demonstrating that conversion is not an active process at the tumor site. The identification of public TCR sequences in intra-tumoral Tregs, also present in the draining lymph node of the same animal, demonstrates that infiltration of tumor tissue by these cells is followed by an intense proliferation in the tumor, which skews the highly diverse TCR repertoire towards a few dominant clones (Sainz-Perez et al, Cancer Res., 2012) We have also demonstrated that the alkylating reagent cyclophosphamide (CTX) can rescue the therapeutic activity of CyaA-E7 against large tumors (Berraondo et al, Cancer Res., 2007). CTX is a widely used chemotherapeutic reagent, which does not induce anti-tumor response by itself, but when injected 24 hours before CyaA-E7, it increases dramatically the expansion of E7 specific CD8+ T cells and eliminates large tumor. It is generally believed that CTX enhances immune responses through the depletion of Treg cells. However, we have demonstrated that after CTX treatment, there is only a temporary and mild decrease of Treg cells in tumor. In contrast, we have observed a recruitment of CD11b+Ly6G+ granulocytes and CD11b+Ly6C+ monocyte to spleen and to tumor, respectively. Interestingly, granulocytes and monocytes are also the major subsets of MDSC. The increase of granulocytes and monocytes along with the expansion of CD8+ T cells indicated that these cells do not suppress immune responses after CTX treatment. Our research interest is to understand the immune responses induced by CTX, focusing on the following directions:
- The qualitative modification of Treg cells after CTX treatment, including phenotypic, functional and genomic analysis.
- The qualitative modification of myeloid cells after CTX treatment. MDSCs represent a group of phenotypically heterogeneous, functionally flexible cells. They could induce inflammatory responses and at same time suppress other immune cells. Tumor may induce the suppressive property of myeloid cells whereas CTX treatment may reprogram these myeloid cells from suppressor to immunogenic cells.
Understanding the responses induced by CTX and how it reprograms immune suppressors is crucial for the design of efficient immunotherapeutic strategies in the future. We are also investigating the efficacy of other chemotherapies to rescue the therapeutic activity of CyaA-E7 against large tumors. In particular, we are analyzing the effect of rapamycin, an inhibitor of Mammalian Target of Rapamycin (mTOR). mTOR is a microenvironment sensor that regulates broad aspects of cellular function, highly implicated in several cancers, including cervical carcinomas, and inhibitors of mTOR, such as rapamycin and its analogs, are extensively evaluated as anti-cancer therapies. Using the TC-1 model, we are also analyzing the role of B cells in anti-tumor immunity and in the escape to therapeutic vaccination. Indeed, it is well established that B cells are able to suppress inflammatory responses during autoimmune diseases. Their immunoregulatory activity is due to their capacity to secrete IL-10, an anti-inflammatory cytokine. Whether they can exert immunosuppressive function in anti-tumor immunity remains unclear. In the TC-1 model, we show that B cells accumulate in the draining LN of tumor-bearing mice and have a different phenotype compared to B cells from naive mice.
Plasmacytoid dendritic cells in tumor immunity
Antigen cross-presentation by plasmacytoid dendritic cell
To induce CD8+ T cell responses against exogenous Ags, antigen presenting cells should deliver exogenous Ag to MHC class I molecules by cross presentation, allowing processing of these Ag in the MHC class I antigen-presentation pathways. We demonstrated that pDCs are able to capture efficiently exogenous Ag at steady state, but are unable to cross present these Ags. However, after activation, pDCs acquired the capacity to cross-present soluble and particular exogenous Ag leading to the generation of efficient primary CD8+ T cell responses against these Ags in vivo (Mouriès et al, Blood, 2008). These results suggest that pDCs could play an important role in the induction of CD8+ T cells responses against virus, which do not infect hematopoeitic cells or tumoral Ags. Furthermore, it is also the first evidence of a regulation of cross presentation by pDCs. The mechanisms of induction of cross-presentation are under investigation.
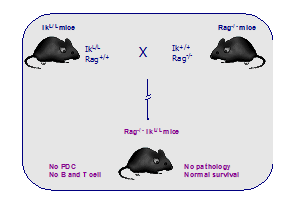
A new mouse model lacking pDCs
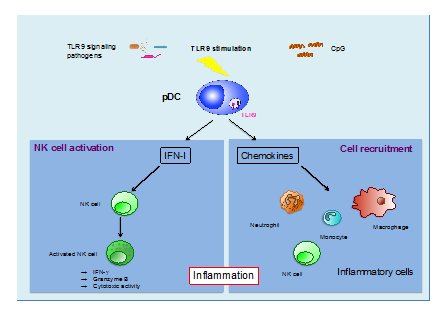
Pivotal role of plasmacytoid dendritic cells in inflammation and NK cell responses
Using our mouse model lacking pDCs, we have established that pDCs are essential for the in vivo induction of NK cell activity in response to TLR9 triggering (Guillerey et al. Blood, 2012). Furthermore, we provided the first evidence that pDCs are critical for the systemic production of a wide variety of chemokines in response to TLR9 activation. Consequently, in the absence of pDCs, we observed a profound alteration in monocytes, macrophages, neutrophils, and NK cell recruitments at the site of inflammation in response to CpG-Dotap. Our results highlight the pivotal role played by pDCs in the induction of innate immune responses following TLR9 triggering.
Conventional but not plasmacytoid dendritic cells foster the systemic virus-induced Type I IFN response needed for efficient CD8 T cell priming
Plasmacytoid dendritic cells (pDCs) are considered to be the principal type-I IFN (IFN-I) source in response to viruses, whereas the contribution of conventional DCs (cDCs) has been underestimated because, on a per-cell basis, they are not considered professional IFN-I-producing cells. Using a non-replicative virus, Baculovirus (BV), we have shown that, despite the high IFN-I-producing abilities of pDCs, in vivo cDCs but not pDCs are the pivotal IFN-I-producers upon viral injection. The supremacy of cDCs over pDCs in fostering the IFN-I response required for CTL activation was also verified in the Lymphocytic Choriomeningitis Virus (LCMV) model. However, when the TLR-independent virus-triggered IFN-I production is impaired, the pDC-induced IFNs-I have a primary impact on CTL activation, as shown by the detrimental effect of pDC depletion and IFN-I-signaling blockade on the residual LCMV-triggered CTL response detected in IPS-1-/- mice (Hervas-Stubbs et al, J. Immunol, 2014).




