Présentation
The use of light from the abyss in life sciences
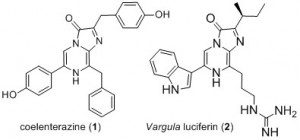
In the last decades, bioluminescent systems based on the expression of a luciferase and the addition of a luciferin to monitor the emission of light have become very important tools for biological investigations. A growing proportion of these systems are using imidazo[1,2‑a]pyrazine luciferins such as the naturally-occurring coelenterazine (1) or the vargula luciferin (2) along with photoproteins or luciferases which came from sea creatures such as Aequorea, Renilla, Gaussia or Oplophorus.
Central to the success of these tools are the synthetic pathways developed over the years to prepare not only these two endogenous substrates, but also analogues endowed with improved bioluminescent signals. And this took place along with extensive alteration of the corresponding luciferases leading to fairly efficient luciferin/luciferase reporting systems. Our contribution to this research domain has been, so far, the design of a new synthetic pathway which provided accesses to even more diverse imidazo[1,2‑a]pyrazines. Some of them turned out to be far more efficient substrates of specific luciferases in terms of signal intensity or duration.
The following figure is illustrating some of our results. It depicts the superposition of the bioluminescence signals obtained with of 57 different imidazo[1,2‑a]pyrazine analogues over two hours, using nanoKAZ, an Oplophorus-derived luciferase. The red curve (Q01) happens to be the signal obtained with furimazine and compound Q108 is among our favorite.
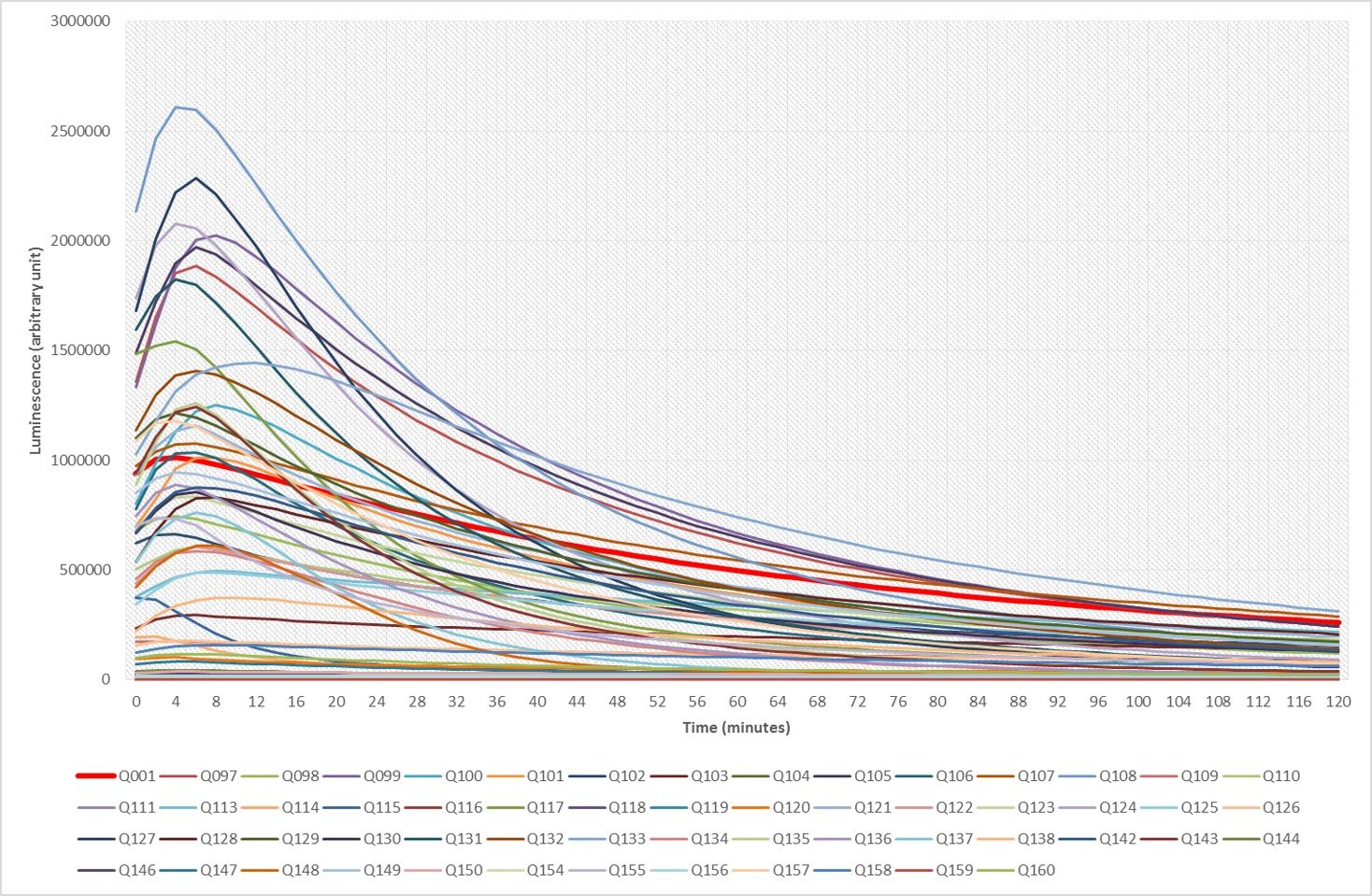
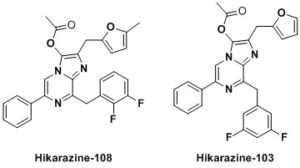
Following a patent [1] many of these results are undergoing the process of being published [2-6]. Moreover, samples of our enhanced luciferins analogues illustrated above can be shipped (at your cost) as stable O-acetylated precursors (Hikarazines) which you will have to hydrolyze to use for your research; do not hesitate to contact us. Concerning Hikarazine-108, you might want to read about its uses in LuLISA-based serological assays [7] which are currently employed to assess the extent of the Covid-19 pandemic in the French population.
In a next stage we are working on two distinct research axis.
The first is the design of small compounds endowed with a red-shifted chemiluminescence but still structurally related to these marine luciferins. The following picture of a 250 mL flask containing one of our chemiluminescent prototype is illustrating where we are now (the emission peaks at 605 nM, but the concentration is very high, FRET-based phenomenon are likely to be involved). Accordingly, such apparent wavelength may well be an artifact as long as we have not designed a luciferase to consume such substances (at a much reduced concentration which will avoid any FRET-based issue). See our recent report, resulting from a collaboration with Horiba Scientific, on an array of analogues [8].

We have been working on “dressing” such compounds with substituents which, for imidazo[1,2‑a] pyrazine-based compounds, are recognized by luciferases (this approach is very much like usual MedChem). From these, if any compounds is displaying a chemiluminescence of interest, we will then alter the luciferases currently using imidazo[1,2‑a]pyrazine luciferins so that these (completely original and eminently patentable) analogues become actual substrates of these enzyme. In case of success this would provide a fully original luciferin/luciferase reporting system endowed with a red (hopefully 605 nM) signal devoid of any prosthetic component such as fluorescent proteins or any other type of rather large constructions.
The second axis is the design of compounds which would provide tools for co-crystallization experiments to get an idea of how the luciferase is catalyzing the oxidation of these luciferins. In this direction we have been quite lucky, stay tuned, this should be published in the future; structure 6TP7, currently on hold, will hopefully be of interest.
One last research project we have been trying to suggest is to undertake the extensive optimization work, which led to very efficient luciferases such as the Oplophorus-derived NanoKAZ/NanoLuc, but starting with other coelenterazine-using luciferases. There are indeed many other bioluminescent and coelenterazine-using organisms in the sea! Again stay tuned, we may have gotten lucky.
The hardest part in all this being (as usual) to get funding (very little luck so far).
Thank you for reading
1. Janin, Y. L.; Coutant, E. P.; Hervin, V.; Gagnot, G.; Jacob, Y.; Goyard, S.; Rose, T. Imidazopyrazine derivatives, process for preparation therof and their uses as luciferins. EP 3395803 / WO 2018197727, 2018.
2. Hervin, V.; Coutant, E. P.; Gagnot, G.; Janin, Y. L., Synthesis of alpha-amino esters via α-nitro or alpha-oxime esters, a review. Synthesis 2017, 49, 4093-4110.
3. Gagnot, G.; Hervin, V.; Coutant, E. P.; Desmons, S.; Baatallah, R.; Monnot, V.; Janin, Y. L., Synthesis of unnatural alpha-amino ethyl esters using ethyl nitroacetate and condensation or cycloaddition reactions. Beilstein J. Org. Chem. 2018, 14, 2846-2852.4.
4. Coutant, E. P.; Hervin, V.; Gagnot, G.; Ford, C.; Baatallah, R.; Janin, Y. L., Unnatural alpha-amino ethyl esters from diethylmalonate or ethyl beta-bromo-alpha-oxime carboxylate. Beilstein J. Org. Chem. 2018, 2853-2859.
5. Coutant, E. P.; Goyard, S.; Hervin, V.; Gagnot, G.; Baatallah, R.; Rose, T.; Jacob, Y.; Janin, Y. L., Gram-scale synthesis of luciferins derived from coelenterazine and original insights in their bioluminescence properties. Org. Biomol. Chem. 2019, 17, 3709-3713.
6. Coutant, E. P.; Gagnot, G.; Hervin, V. O.; Baatallah, R.; Goyard, S.; Jacob, Y.; Rose, T.; Janin, Y. L. Bioluminescence Profiling of NanoKAZ/NanoLuc Luciferase Using a Chemical Library of Coelenterazine Analogues. Chemistry 2020, 26, 948-958.
7. Goyard, S.; Balbino, B.; Chinthrajah, R. S.; Lyu, S.-C.; Janin, Y. L.; Bruhns, P.; Poncet, P.; Galli, S. J.; Nadeau, K. C.; Reber, L. L.; Rose, T. A highly sensitive bioluminescent method for measuring allergen-specific IgE in microliter samples. Allergy 2020, DOI: 10.1111/all.14365
8. Gagnot, G.; Hervin, V.; Coutant, E. P.; Goyard, S.; Jacob, Y.; Rose, T.; Hibti, F. E.; Quatela, A.; Janin, Y. L. Core-modified coelenterazine luciferin analogues, synthesis and chemiluminescence properties. Chem. Eur. J. 2020, doi.org/10.1002/chem.202004311.