Link to Pubmed [PMID] – 29946619
Nanoscale. 2018 Jul 9;10(26):12743-12753.
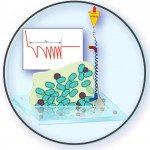
The safe use and design of nanoparticles (NPs) ask for a comprehensive interpretation of their potentially adverse effects on (micro)organisms. In this respect, the prior assessment of the interactions experienced by NPs in the vicinity of – and in contact with – complex biological surfaces is mandatory. It requires the development of suitable techniques for deciphering the processes that govern nano-bio interactions when a single organism is exposed to an extremely low dose of NPs. Here, we used atomic force spectroscopy (AFM)-based force measurements to investigate at the nanoscale the interactions between carboxylate-terminated polyamidoamine (PAMAM) nanodendrimers (radius ca. 4.5 nm) and two bacteria with very distinct surface properties, Escherichia coli and Lactococcus lactis. The zwitterionic nanodendrimers exhibit a negative peripheral surface charge and/or a positive intraparticulate core depending on the solution pH and salt concentration. Following an original strategy according to which a single dendrimer NP is grafted at the very apex of the AFM tip, the density and localization of NP binding sites are probed at the surface of E. coli and L. lactis mutants expressing different cell surface structures (presence/absence of the O-antigen of the lipopolysaccharides (LPS) or of a polysaccharide pellicle). In line with electrokinetic analysis, AFM force measurements evidence that adhesion of NPs onto pellicle-decorated L. lactis is governed by their underlying electrostatic interactions as controlled by the pH-dependent charge of the peripheral and internal NP components, and the negatively-charged cell surface. In contrast, the presence of the O-antigen on E. coli systematically suppresses the adhesion of nanodendrimers onto cells, may the apparent NP surface charge be determined by the peripheral carboxylate groups or by the internal amine functions. Altogether, this work highlights the differentiated roles played by surface polysaccharides in mediating NP attachment to Gram-positive and Gram-negative bacteria. It further demonstrates that the assessment of NP bioadhesion features requires a critical analysis of the electrostatic contributions stemming from the various structures composing the stratified cell envelope, and those originating from the bulk and surface NP components. The joint use of electrokinetics and AFM provides a valuable option for rapidly addressing the binding propensity of NPs to microorganisms, as urgently needed in NP risk assessments.