Link to Pubmed [PMID] – 23980063
Blood 2013 Oct;122(18):3160-4
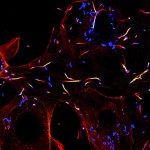
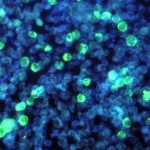
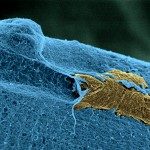
Tumor engraftment followed by monoclonal antibody (mAb) therapy targeting tumor antigens represents a gold standard for assessing the efficiency of mAbs to eliminate tumor cells. Mouse models have demonstrated that receptors for the Fc portion of immunoglobulin G (FcγRs) are critical determinants of mAb therapeutic efficacy, but the FcγR-expressing cell populations responsible remain elusive. We show that neutrophils are responsible for mAb-induced therapy of both subcutaneous syngeneic melanoma and human breast cancer xenografts. mAb-induced tumor reduction, abolished in neutropenic mice, could be restored in FcγR-deficient hosts upon transfer of FcγR+ neutrophils or upon human FcγRIIA/CD32A transgenic expression. Finally, conditional knockout mice unable to perform FcγR-mediated activation and phagocytosis specifically in neutrophils were resistant to mAb-induced therapy. Our work suggests that neutrophils are necessary and sufficient for mAb-induced therapy of subcutaneous tumors, and represent a new and critical focal point for optimizing mAb-induced immunotherapies that will impact on human cancer treatment.