Link to Pubmed [PMID] – 26865269
Link to DOI – 10.1002/eji.201546132
Eur J Immunol 2016 05; 46(5): 1291-9
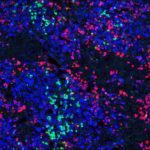
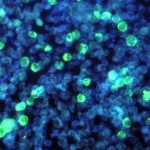
Humanized mice harboring human immune systems (HIS) represent a platform to study immune responses against pathogens and to screen vaccine candidates and novel immunotherapeutics. Innate and adaptive immune responses are suboptimal in HIS mice, possibly due to poor reconstitution of human antigen-presenting cells, including dendritic cells (DCs). DC homeostasis is regulated by cytokine availability, and Flt3-ligand (Flt3L) is one factor that conditions this process. Mouse myelopoiesis is essentially normal in most current HIS models. As such, developing mouse myeloid cells may limit human DC reconstitution by reducing available Flt3L and by cellular competition for specific “niches.” To address these issues, we created a novel HIS model that compromises host myeloid cell development via deficiency in the receptor tyrosine kinase Flk2/Flt3. In Balb/c Rag2(-/-) Il2rg(-/-) Flt3(-/-) (BRGF) recipients, human conventional DCs and plasmacytoid DCs develop from hCD34(+) precursors and can be specifically boosted with exogenous Flt3L. Human DCs that develop in this context normally respond to TLR stimulation, and improved human DC homeostasis is associated with increased numbers of human NK and T cells. This new HIS-DC model should provide a means to dissect human DC differentiation and represents a novel platform to screen immune adjuvants and DC targeting therapies.