About
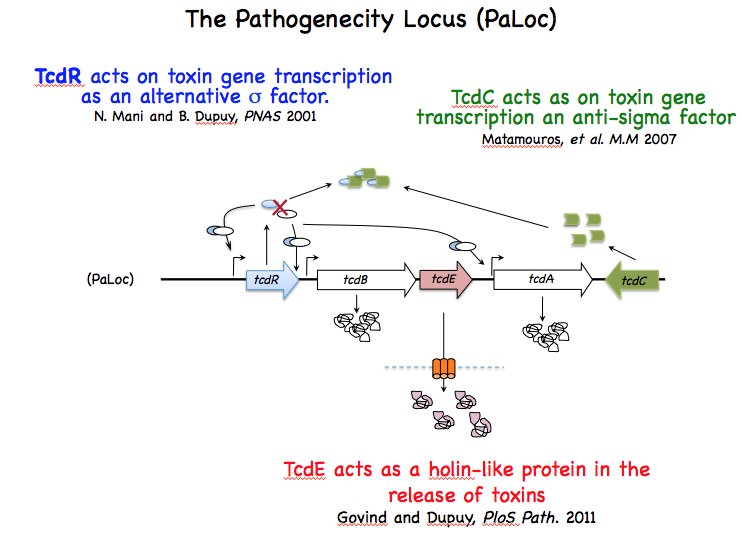
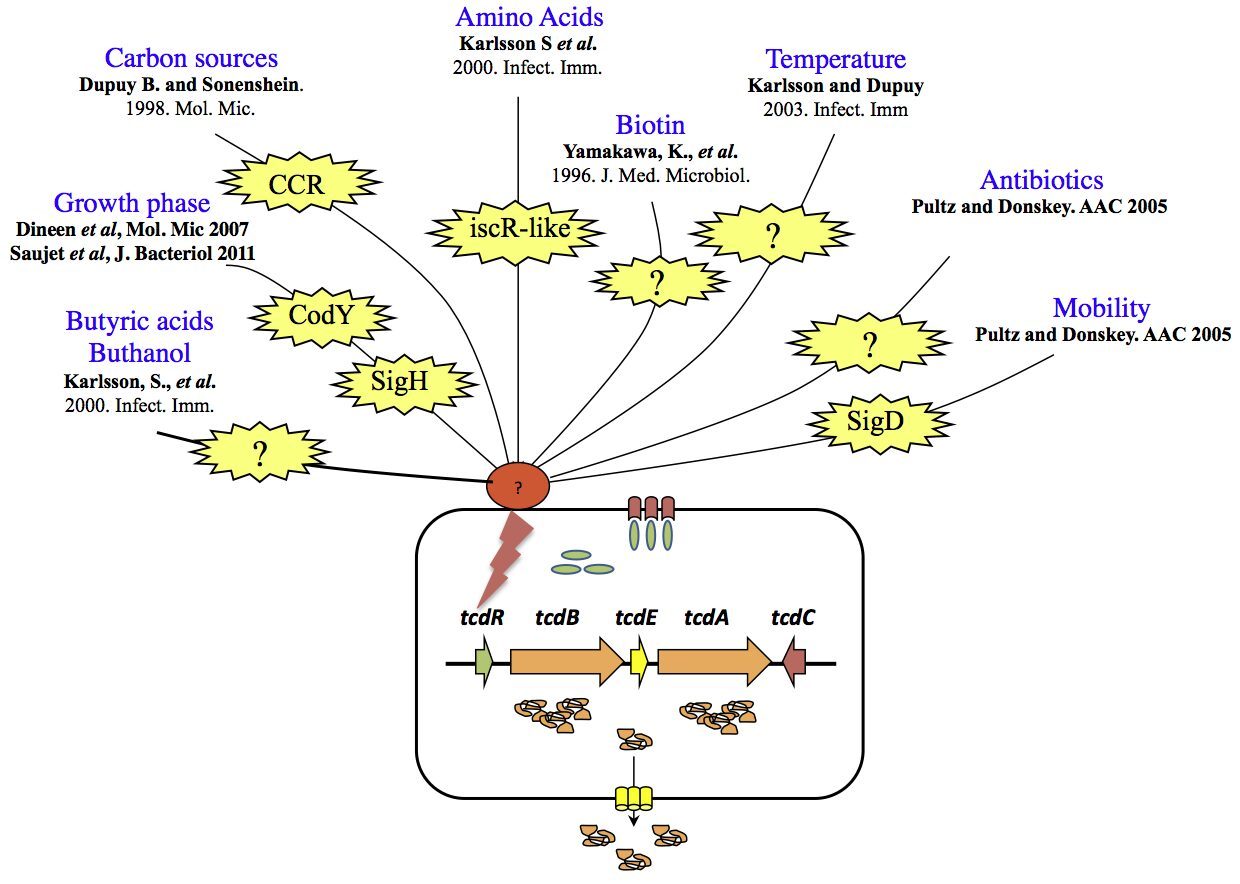
The variable spectrum of diseases caused by C. difficile (CD) is correlated for the severe forms to the level of toxins produced during host infection. This strengthened the idea that regulation of toxin synthesis is an important part of CD pathogenesis. A breakthrough in understanding regulation of toxin gene expression came when we found that together with three accessory genes (tcdR, tcdE and tcdC), toxin genes (tcdA and tcdB) form a chromosomal genetic unit of 19.6 kb designated the pathogenicity locus (PaLoc), which is only present in toxin-producing strains. TcdR is an alternative s factor that interacts with the core enzyme of the RNA polymerase (RNAP) to specifically recognize and activate transcription of toxin genes. TcdR was the first member of a new group of sigma factors within the s70 family, which currently contains several sigma factors of toxinogenic clostridia. TcdC is an anti-sigma factor that negatively regulates toxin gene expression by interfering with the ability of the TcdR-containing RNAP to recognize the tcdA and tcdB promoters. Finally, TcdE protein encoded by the last PaLoc gene presents structural features in common with bacteriophage holins involved in phage release. CD toxins have no export signature and their secretion is not explained by cell lysis. We demonstrated that CD toxin secretion requires holin activity of TcdE but without induction of cell lysis contrary to the classical phage holins . TcdE was the first example of a bacterial protein that release toxins by a phage-like system, an unusual secretion mechanism. Other members of the lar