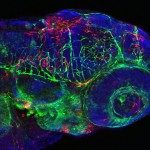
About
Emma Colucci-Guyon, Jean-Pierre Levraud, Laurent Boucontet, Milka Sarris, Maxence Fretaud
The early control of potentially invading microbes by our immune system primarily depends on its main professional phagocytes – macrophages and neutrophils. While the different functions of these two cell types have been extensively studied, little is known about their actual respective contributions to the initial control of invading microorganisms, before the onset of adaptive immune responses. The zebrafish immune system develops gradually: its adaptive arm becomes operational -in terms of ability to mount an antibody response- only when the larva develops into a juvenile fish. Thus, the larva has a purely innate immune system, consisting in macrophages and neutrophils.
 Movie 1. Three hours following intra-venous injection of B. subtilis bacteria in a zebrafish embryo by 30 hpf, all bacteria have been engulfed by the primitive macrophages – each macrophage gathering many bacteria in one big phagosome; towards the end of the movie, the central macrophage also engulfs a yet healthy erythroblast (real-time live video-enhanced DIC/Nomarski microscopy).
Movie 1. Three hours following intra-venous injection of B. subtilis bacteria in a zebrafish embryo by 30 hpf, all bacteria have been engulfed by the primitive macrophages – each macrophage gathering many bacteria in one big phagosome; towards the end of the movie, the central macrophage also engulfs a yet healthy erythroblast (real-time live video-enhanced DIC/Nomarski microscopy).
Through in vivo imaging in zebrafish larvae, we found that although macrophages and neutrophils are equally attracted to inoculated bacteria (Le Guyader et al, 2008), surprisingly the contribution of the two phagocytes to microbe engulfment and elimination differ radically according to the site of microbe inoculation. While macrophages very efficiently engulf fluid-borne bacteria (in blood – Movie 1 – or in fluid-filled body cavities, such as pericardial or brain ventricles), neutrophils barely do so. In contrast, they very efficiently engulf and eliminate microbes presented on surfaces within tissues, sweeping them up as they move over them, in a ‘vacuum-cleaner’ type of behaviour (Colucci-Guyon et al, 2011) (Figure 1 and Movie 2). This underappreciated surface-dependent phagocytosis of unopsonized microbes is most likely crucial in the early stages of microbe invasion in any vertebrate host. We are now studying the host’s innate immune response to pathogenic microbes of medical interest, in collaboration with Institut Pasteur units expert in these pathogens. At the molecular level, we also investigated how chemoattractant signals such as chemokines can guide the migration of leukocytes within complex living tissues, where these cells are exposed to myriads of signals at the same time. To this end, we established in vivo assays to analyse how chemokines diffuse and become presented in tissue environments and the mechanisms by which they influence the constitutively high motility of leukocytes (Sarris et al. 2012).
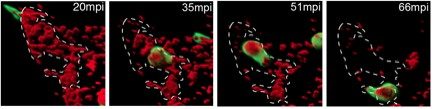
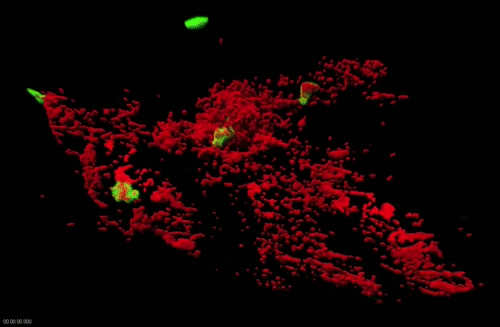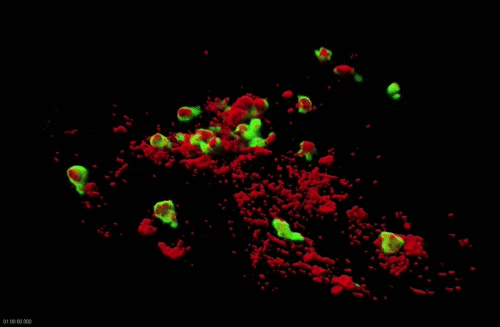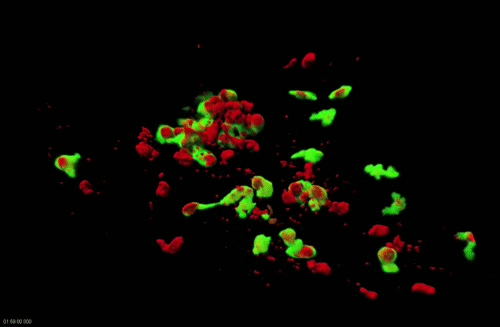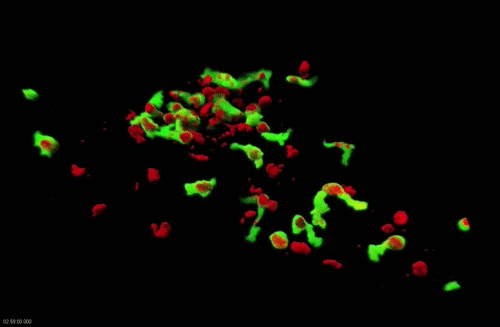 Movie 2. Dynamics of surface-associated phagocytosis of bacteria by neutrophils in vivo. Red fluorescent bacteria (E.coli) were injected subcutaneously in a zebrafish swimming larva harboring green fluorescent neutrophils. Live confocal fluorescence imaging was performed every min. from 20 to 200 min post injection.
Movie 2. Dynamics of surface-associated phagocytosis of bacteria by neutrophils in vivo. Red fluorescent bacteria (E.coli) were injected subcutaneously in a zebrafish swimming larva harboring green fluorescent neutrophils. Live confocal fluorescence imaging was performed every min. from 20 to 200 min post injection.
Collaborations : Roland Brosch (Unité Pathogénomique mycobactérienne intégrée, IP); Pascale Cossart (Unité des Interactions Bactéries-Cellules, IP), Serge Mostowy (Section of Microbiology, Imperial College, London, UK) : Probing the role of autophagy in antimicrobial defense in vivo using zebrafish; Jean-Marc Ghigo (Unité Génétique des Biofilms) : Molecular analysis of protective interferences in a zebrafish model of bacterial intestinal colonization; Véronique Le Cabec & Isabelle Maridonneau-Parini (IBPS, Toulouse): Trim33 role in macrophage amoeboid motility.