Pierre Lafaye is head of the Antibody Engineering Platform at Pasteur Institute. I completed a Pharmaceutical degree at Bordeaux University then moving on to specialize in immunology at Pasteur Institute. I also defended my PhD at the same organization before completing a post-doctoral training at the US Army Medical Research Institute of Infectious Diseases (USAMRIID), Fort Detrick, Maryland. I have worked there on cloning and expression of snake toxin genes. I then moved back to the Pasteur Institute once again in 1991 to conduct research on human recombinant antibodies.
In 2003, I was involved in the study of research on homodimeric antibodies, analyzing the genomic organization of llama antibodies gene. We have shown that a single IgH locus contains all of the genetic elements required for the generation of the two types of Igs. The alpaca IgH locus is composed of a V region that contains both VHH and VH genes followed by a unique DH-JH cluster and C region genes, which include both CHH and CH genes. Although this general gene organization greatly resembles that of other typical mammalian Vn-Dn-Jn-Cn translocon IgH loci, the intermixed gene organization within the alpaca V and C regions reveals a new type of translocon IgH locus (Achour et al, 2008).
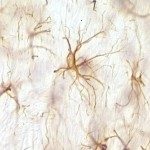
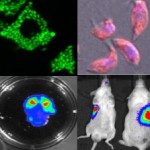
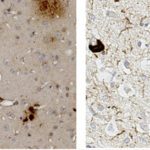
In 2010, I join the PFIA to continue to work on VHHs. I have also demonstrated that VHHs are able to cross the Blood-Brain Barrier (BBB) and to bind GFAP, an intracellular antigen (Li et al, 2012). According to these results, we have been funded by Roche for 4 years for the development of VHH for the in vivo diagnosis of Alzheimer’s disease (AD). We have raised VHHs that recognize amyloid deposits and intracellular tau neurofibrillary tangles (NFTs), the two core lesions of AD. These VHHs are naturally able to cross the BBB following intra-venous injections in live mice. They can diffuse in cerebral tissue, penetrate into neurons, survive in the cytoplasm and label specifically amyloid plaques and intraneuronal NFTs (Li et al, 2016, Vandesquille et al, 2017). These findings constitute strong evidence of transmigration of VHH through the BBB and through cell membranes to label intracellular antigens, opening new developments for in vivo brain imaging approaches.
Several projects raised in collaboration with academic partners (pasteurian or not) are based on these unique properties and they allow us to explore the potentiality of VHHs (i.e. imaging of intracerebral targets, carrier for the delivery of molecules through the BBB, disrupting virus/host pathogenic interaction networks, diagnosis of infectious diseases). These projects are usually funded via in-house or national calls.