Cliquez pour voir le graph
Connexions
Présentation
Membres

Timothy Wai
Responsable de Structure
Responsable
Anciens Membres
Projets
Projet Transversal
Financements
Publications
Télécharger-
2024Is mitochondrial morphology important for cellular physiology?, Trends Endocrinol Metab 2024 Oct; 35(10): 854-871.
-
2024Dynamic label-free analysis of SARS-CoV-2 infection reveals virus-induced subcellular remodeling., Nat Commun 2024 Jun; 15(1): 4996.
-
2023Mtfp1 ablation enhances mitochondrial respiration and protects against hepatic steatosis., Nat Commun 2023 Dec; 14(1): 8474.
-
2023MPC2 variants disrupt mitochondrial pyruvate metabolism and cause an early-onset mitochondriopathy., Brain 2023 Mar; 146(3): 858-864.
-
2023Mitochondrial dynamics and metabolic regulation control T cell fate in the thymus., Front Immunol 2023 ; 14(): 1270268.
-
2022Mitochondrial Fission Process 1 controls inner membrane integrity and protects against heart failure., Nat Commun 2022 Nov; 13(1): 6634.
-
2022The mitochondrial seryl-tRNA synthetase SARS2 modifies onset in spastic paraplegia type 4., Genet Med 2022 Nov; 24(11): 2308-2317.
-
2022CHCHD10 and SLP2 control the stability of the PHB complex: a key factor for motor neuron viability., Brain 2022 Oct; 145(10): 3415-3430.
-
2022FGF21 modulates mitochondrial stress response in cardiomyocytes only under mild mitochondrial dysfunction., Sci Adv 2022 Apr; 8(14): eabn7105.
-
2021Mitochondrial respiration restricts Listeria monocytogenes infection by slowing down host cell receptor recycling., Cell Rep 2021 Nov; 37(6): 109989.
-
+Voir la liste complète de publications
Images & Médias
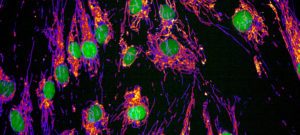
Credit : Yining ZHAO
Contact
Timothy Wai timothy.wai@pasteur.fr Institut Pasteur 25-28 Rue du Docteur Roux 75015