Lien vers Pubmed [PMID] – 19546195
Lien DOI – 10.1128/IAI.00408-09
Infect Immun 2009 Sep; 77(9): 3651-60
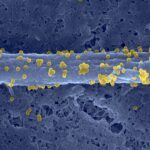
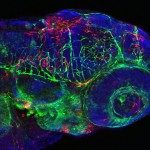
The zebrafish, Danio rerio, has become a popular vertebrate model for the study of infections, mainly because of its excellent optical accessibility at the embryonic and larval stages, when the innate immune system is already effective. We have thus tested the susceptibility of zebrafish larvae to the human pathogen Listeria monocytogenes, a gram-positive, facultative, intracellular bacterium that is known to survive and multiply in professional phagocytes and that causes fatal meningitis and abortions. Intravenous injection of early zebrafish larvae resulted in a progressive and ultimately fatal infection. Blood-borne L. monocytogenes bacteria were quickly trapped and engulfed by macrophages, an event that, for the first time, could be captured in vivo and in real time. Granulocytes also participated in the innate immune response. As in mammals, bacteria could escape the macrophage phagosome in a listeriolysin-dependent manner and accessed the cytosol; this event was critical for bacterial virulence, as listeriolysin-deficient bacteria were completely avirulent. Actin comet tails and protrusions were observed, suggesting cell-to-cell spread; these phenomena also played a role in virulence in zebrafish larvae, as actA-deficient bacteria were attenuated. These results demonstrate the relevance of the genetically tractable and optically accessible zebrafish model for the study of L. monocytogenes pathogenesis and particularly for the dissection of its interactions with phagocytes in vivo, a key factor of L. monocytogenes virulence.