Présentation
Mucosal transmission of HIV-1 can induce a local production of IgG and IgA antibodies predominantly targeting the gp41 subunit of the viral envelope glycoprotein gp160. However, whether they limit viral dissemination upon HIV-1 exposure is unclear. Moreover, polyreactive antibodies naturally produced by intestinal B cells and coating commensals in situ have been proposed to compromise optimal humoral responses to HIV-1 by immune diversion but overall, very little is known about the antibody response to HIV-1 at mucosal sites and the properties of gut-resident B cells recognizing the virus.
To interrogate the intestinal B cells “sensing” HIV-1, we probed 76 recombinant monoclonal antibodies expressed from gp160-binding IgA+ and IgG+ B cells from rectosigmoid colon tissues of HIV-1-infected individuals. We found that a large fraction of mucosal mAbs were polyreactive, and showed only low-affinity to HIV-1 envelope glycoproteins particularly, the gp41 moiety. A few high-affinity gp160 mAbs were isolated but lacked neutralizing, potent ADCC and transcytosis-blocking capacities. Instead, they displayed cross-reactivity with defined self-antigens. Specifically, certain intestinal HIV-1 gp41 mAbs cross-reacted with the p38α mitogen-activated protein kinase 14 (MAPK14) (Planchais et al, 2019).
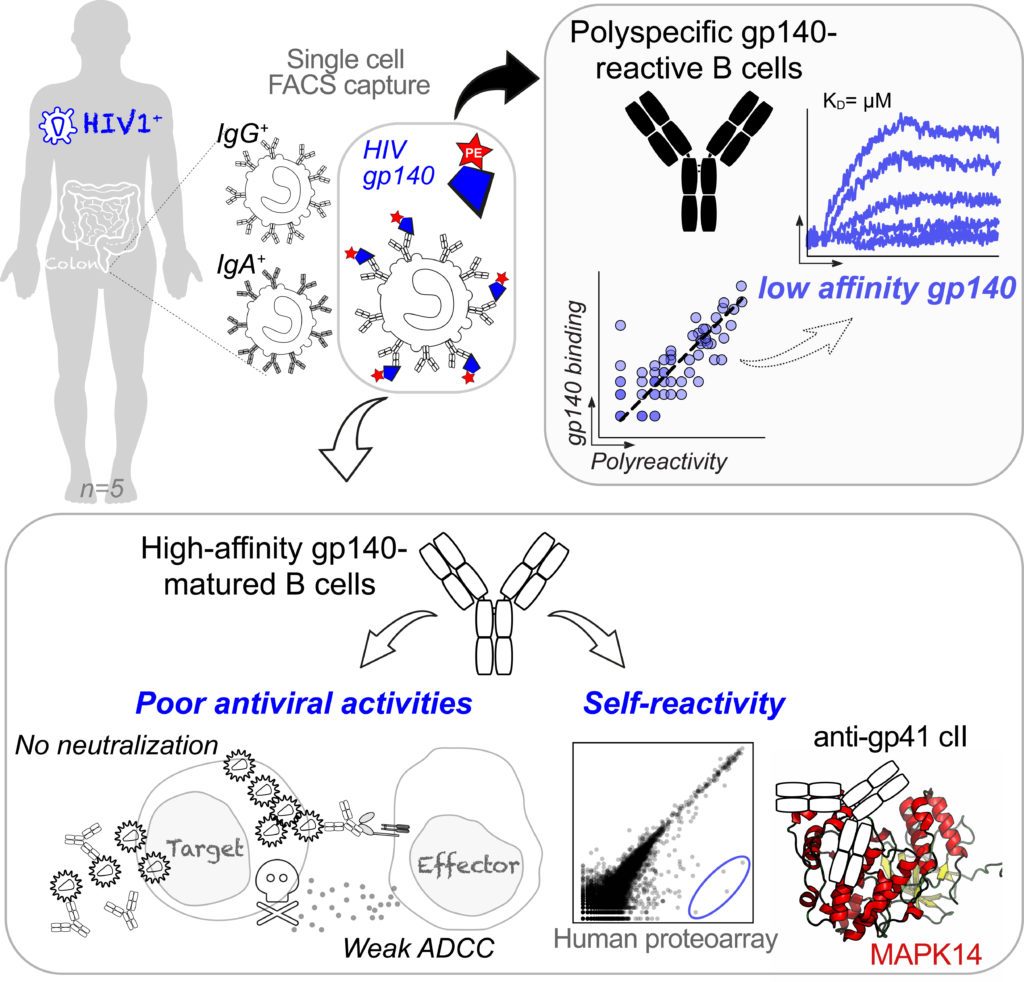
Altogether, our data suggest that the physiologic polyreactivity of intestinal B cells and molecular mimicry-based self-reactivity of HIV-1 antibodies are two phenomena likely diverting and/or impairing mucosal humoral immunity to HIV-1. Our data suggests an inability of the gut immune system to locally generate functional high-affinity antibodies in response to HIV-1 infection.
In a follow-up project funded by Sidaction, and in collaboration with Drs Laurent Hocqueloux (Service des Maladies Infectieuses et Tropicales, CHR d’Orléans-La Source) et Sophie Huë (Service d’Immunologie Biologique, Hôpital Henri Mondor), we aim at understanding whether the timing of the antiretroviral therapy (ART) for AIDS treatment can positively influence B-cell / antibody responses at mucosal surfaces in seropositive patients as we previously suggested (Planchais et al, 2018).
It has become increasingly evident that besides their therapeutic potential, decoding the B-cell developmental pathways of HIV-1 bNAbs would provide blueprints for the design of effective vaccine strategies. All bNAbs identified and characterized so far were naturally produced as IgG molecules, yet whether IgA-expressing B cells with antiviral properties can be induced in response to HIV-1 infection has never been addressed. Our research is tackling this issue.
More globally, IgA antibodies play a key protective function as immune effectors against invading pathogens but also as immunosuppressors of pro-inflammatory responses, and immunomodulators of the gut microbiota. Conversely, IgA-antigen complexes can promote inflammatory processes, and therefore be pathogenic mediators in certain auto-inflammatory and autoimmune disorders. In humans, besides their paramount presence in mucosal tissues, IgA class-switched (IgA+) memory B cells and IgA antibodies are abundant in the blood1. Although circulating IgA+ memory B cells and their corresponding secreted immunoglobulins likely possess major protective and/or regulatory immune roles, little is known about their specificity and function. Hence, we were interested in characterizing blood IgA memory B-cell antibodies in normal conditions in healthy humans as a first step, prior to their study in infectious diseases. To this end, we cloned, produced and characterized the gene repertoire and reactivity of more than 330 IgA and IgG memory B-cell antibodies from healthy donors (Lorin et al, 2015; Prigent et al, 2016). We found that although IgA+ and IgG+ memory antibodies shared common immunoglobulin gene features, IgA+ memory B cells contained fewer polyreactive clones and importantly, only rare self-reactive clones compared to IgG+ memory B cells. We further showed that IgA self-reactivity was acquired following B-cell affinity maturation and not antibody class switching.
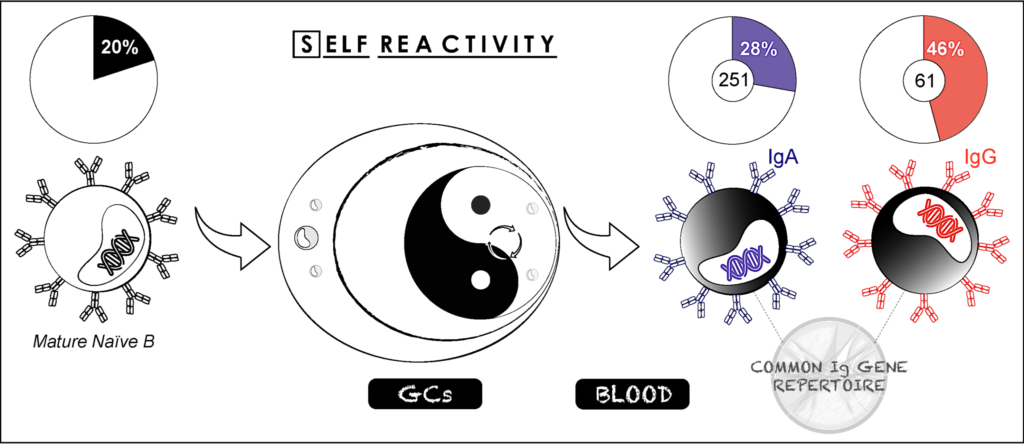 Together, our data show that although blood class-switched CD27+IgG+ and CD27+IgA+ B cells derive from the GC-dependent pathway, and are often considered as a relatively homogenous memory B-cell population as key components of the “reactive” humoral immunity, they differ considerably regarding their level of poly- and self-reactivity. This suggests the existence of different maturation pathways and/or different regulatory mechanisms for removing autoreactive clones from the IgG+ and IgA+ memory B-cell repertoires (Prigent et al, 2016). Strikingly, the later may provide an attractive explanation for the rarity of IgA-mediated autoimmune diseases (i.e., coeliac disease and IgA blistering dermatoses), whereas pathogenic and non-pathogenic IgG autoantibodies are a hallmark for most autoimmune disorders.
Together, our data show that although blood class-switched CD27+IgG+ and CD27+IgA+ B cells derive from the GC-dependent pathway, and are often considered as a relatively homogenous memory B-cell population as key components of the “reactive” humoral immunity, they differ considerably regarding their level of poly- and self-reactivity. This suggests the existence of different maturation pathways and/or different regulatory mechanisms for removing autoreactive clones from the IgG+ and IgA+ memory B-cell repertoires (Prigent et al, 2016). Strikingly, the later may provide an attractive explanation for the rarity of IgA-mediated autoimmune diseases (i.e., coeliac disease and IgA blistering dermatoses), whereas pathogenic and non-pathogenic IgG autoantibodies are a hallmark for most autoimmune disorders.