RESEARCH STATEMENT
I became fascinated by RNAi and related RNA silencing phenomena during my graduate studies. Driven by my strong interest in epigenetic phenomena, I joined the laboratory of Dr. Alla Grishok at Columbia University, where I used C. elegansas a model system, and used genomic and biochemical approaches to elucidate the functions of chromatin-binding proteins and small RNAs in gene regulation. I discovered an unprecedented role of a specific class of small RNAs in global transcriptional activation and chromatin organization (Cecere et al., 2014). Our work revealed a new paradigm in transcriptional regulation, and changed the long-standing dogmatic view of RNAi as simply adapted to silence gene expression. Instead, our findings advanced the concept that nuclear Argonaute may constitute an RNAi-based mechanism to regulate the epigenetic and transcriptional landscapes during cell divisions or across multiple generations.
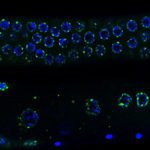
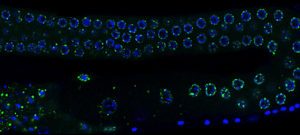
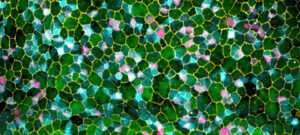
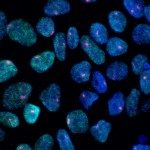
When I started my laboratory at Institut Pasteur in January 2016, I decided to tackle new and emerging fundamental questions in the epigenetic field, such as the capacity of small RNA to transmit information across generations. I decided to address the long-standing question of the cause of the transgenerational progressive loss of fertility in piRNA mutants. Our results demonstrated that this transgenerational phenotype is not caused by de-repression of repetitive elements, as previously thought, but rather by the epigenetic silencing of all the replicative histone genes (Barucci et al., 2020). Therefore, our study helped to change the current dogma that upregulation of repetitive elements underlies the sterility in piRNA mutants. In addition, we demonstrated that the transmission of a pool of small RNAs antisense to histone genes into wild-type worms also epigenetically affects their fertility. Overall, our discoveries advance the concept that small RNAs serve as epigenetic molecules that transmit traits across generations. In addition, we have dissected the molecular mechanism by which small RNAs antisense to histone genes are generated and transmitted across generations in piRNA mutants. These findings were recently published in Nature Cell Biology. Inherited small RNAs can also have essential developmental functions.
In another recent work (Quarato et al., 2020), we demonstrated an essential role of a catalytically active Argonaute protein (CSR-1) and its interacting small RNAs in maternal mRNA clearance during maternal to zygotic transition. This maternally inherited Argonaute is capable of cleaving maternal mRNAs that are no longer engaged in translation in the cytoplasm of somatic blastomeres. Therefore, the germline produces small RNAs that trigger the degradation of the complementary maternal mRNAs in the embryo. This mechanism might be generally conserved to clear maternal mRNAs during the maternal to zygotic transition. Given our scientific approaches and my longstanding interest in small RNA biology, I am confident that I will be able to efficiently solve new fundamental questions such as how endogenous small RNAs are generated and inherited across different tissues or across generations and their mechanism of action.
EDUCATION
2008 PhD in Human Biology and Genetics, University of Rome “La Sapienza”, Department of Cellular Biotechnology and Hematology (BCE), Italy.
Research Project: Molecular dissection of post-transcriptional gene silencing pathways in Neurospora crassa.Supervisor:Prof. Carlo Cogoni
2004 MSc in Biotechnology, University Federico IIof Naples, Italy
Research Project: Molecular mechanisms of interaction between plant and antagonistic fungi.Supervisor:Prof. Matteo Lorito
CURRENTPOSITION(S)
2015 – present Group Leader, Department of Development and Stem Cell Biology, Institut Pasteur, Paris, FRANCE
PREVIOUSPOSITIONS
2011 – 2015 Associate Research Scientist, Department of Biochemistry and Molecular Biophysics, Columbia University Medical Center, USA
2008 – 2011 Postdoctoral Research Scientist, Department of Biochemistry and Molecular Biophysics, Columbia University Medical Center, USA
FELLOWSHIPS AND AWARDS
2013 Keystone Symposia Scholarship Award. Keystone Symposia: RNA Silencing. Whistler, British Columbia Canada.
2007 Best PhD thesis, Centre of Excellence for Biology and Molecular Medicine (BEMM).
2004 – 2008 Doctoral Fellowship, Graduate School of “La Sapienza” University of Rome
SUPERVISION OF GRADUATE STUDENTS AND POSTDOCTORAL FELLOWS
2016 – 2019 2 Postdocs/ 2 PhD/ 4 Master Students
Department of Development and Stem Cell Biology, Institut Pasteur, Paris, FRANCE
TEACHING ACTIVITIES
2015 – to date Lecturer in:M2 program, Universite Paris-Saclay; M2 program, University Paris-Diderot; M1 program, École Normale Supérieure; Ecole doctorale, Muséum National d’Histoire Naturelle;Molecular Biology of the Cell course at Institut Pasteur
2019 Director of the MOOC on epigenetics, Institut Pasteur
ORGANISATION OF SCIENTIFIC MEETINGS
2019 Abcam conference “RNA in epigenetic and chromatin regulation”. Institut Pasteur.Organizer
REVIEWING ACTIVITIES
2019 Member of the jury auditions blanches Aviesan ERC CoG
2019 – presentAssociate editor for Frontiers in molecular biosciences
2014 – 2019 Reviewer for Nature Communications, Communication biology, eLIFE, Nucleic Acid Research, Cell System, EMBO Journal.
2014 – 2019 Associate Grant evaluation for ERC, Welcome Trust, HFSP
TALKS AT CONFERENCES
2018 Keynote speaker at Young Embryologist Network Conference. The Francis Crick Institute, London, UK.Invited
2018 EMBO workshop on piRNAs and PIWI proteins. Montpellier, France. Selected
2017 VerMidi XX meeting. ENS Lyon. Selected
2017 C. elegans – CRISPR, RNAi and genetics courses. CRG, Barcellona, ES. Invited
2017 9thBordeaux RNA club Symposium. Bordeaux, France.Invited
2016 AVIESAN meeting “RNA as an adaptor guiding molecular processes”. Paris, France. Invited
2015 Transgenerational Epigenetic Inheritance Workshop. West Sussex, UK. Selected
2015 20thInternational C. elegans Meeting, UCLA, Los Angeles, USA. Selected
2015 20th Annual Meeting of the RNA Society, Madison, Wisconsin. Selected
2014 Cold Spring Harbor Laboratory Meeting: Regulatory & Non-Coding RNAs, NY, USA. Selected
2013 New York Area Worm Meeting (NYAWM), City University of New York, New York, USA. Invited
2013 19th International C. elegans Meeting, UCLA, Los Angeles, USA. Selected
2013 Columbia Stem Cell Initiative. Columbia University. New York, USA. Invited
2012 Cold Spring Harbor Laboratory Meeting: Regulatory & Non-Coding RNAs. NY, USA. Selected
2011 Keystone Symposia: Mechanism and Biology of Silencing. Monterey, USA. Selected
2009 New York Academy of Science (NYAS). RNAi discussion group. New York, USA.Invited
2007 Italian Federation of Life Science (FISV) congress. Riva del Garda, Italy. Selected
INVITED SEMINARS
2019 Institute History and Philosophy Science and Technical (IHPST), Sorbonne University. Paris, France.
2019 “InteRNAt” Summer school on small RNAs. Sete, France.
2019 EMBL, Departmental seminar. Monterotondo, Italy.
2014 Institut de Biologie de l’Ecole Normale Supérieure (IBENS), Departmental seminar.
2014 Max Plank Institute for Molecular Genetics (MPIMG). Departmental seminar. Berlin, Germany.
2012 Columbia University. Departmental seminar. New York, USA.
2007 Centre of Excellence for Biology and Molecular Medicine (BEMM). Rome, Italy.
AWARDS
2013 Keystone Symposia Scholarship Award. Keystone Symposia: RNA Silencing. Whistler, British Columbia Canada.
2007 Best PhD thesis, Centre of Excellence for Biology and Molecular Medicine (BEMM).
2004 Doctoral Fellowship, Graduate School of “La Sapienza” University of Rome.
PUBLICATIONS
- Quarato, P., Singh, M., Cornes, E., Li, B., Bourdon, L., Mueller, F., Didier, C., Cecere, G*. Germline inherited small RNAs facilitate the clearance of untranslated maternal mRNAs in C. elegans embryos. Nature Communications. Mar 4;12(1):1441. doi: 10.1038/s41467-021-21691-6 *corresponding author
- Barucci, G., Cornes, E., Singh, M., Li, B., Ugolini, M., Samolygo, A., Didier, C., Dingli, F., Loew, D., Quarato, P., Cecere, G.*. Small RNA-mediated transgenerational silencing of histone genes impairs fertility in piRNA mutants. Nature Cell Biology. 2020 Feb doi:10.1038/s41556-020-0462-7 (Featured as the issue cover) *corresponding author
- Serrat X, Kukhtar D, Cornes E, Esteve-Codina A, Benlloch H, Cecere G, Cerón J. CRISPR editing of sftb-1/SF3B1 in Caenorhabditis elegans allows the identification of synthetic interactions with cancer-related mutations and the chemical inhibition of splicing. PLoS Genetics.2019 Oct 21;15(10). (Collaborative project with the Ceron Lab)
- Cecere, G,*and Grishok, A.RNA Chromatin Immunoprecipitation (RNA-ChIP) in Caenorhabditis elegans. Bio-protocol. 2014 Dec20 ;4(24). doi: 10.21769/BioProtoc.1358. *corresponding author
- Cecere, G., Hoersch, S., O’Keeffe, S., Sachidanandam, R. and Grishok, A. Global effects of the CSR-1 RNA interference pathway on the transcriptional landscape. Nature structural & molecular biology. 2014 Apr;21(4):358-65. doi: 10.1038/nsmb.2801. (Comment in Nat Rev Mol Cell Biol. 2014)
- Cecere, G.and Grishok, A. A nuclear perspective on RNAi pathways in metazoans. Biochimica et biophysica acta. 2014 Mar;1839(3):223-33. doi: 10.1016/j.bbagrm.2013.11.009 Review.
- Cecere, G., Hoersch, S., Jensen, M. B., Dixit, S. and Grishok, A. The ZFP-1(AF10)/DOT-1 Complex Opposes H2B Ubiquitination to Reduce Pol II Transcription. Molecular cell. 2013 Jun 27;50(6):894-907. doi: 10.1016/j.molcel.2013.06.002.
- Avgousti D.C., Cecere G., Grishok A.The conserved PHD1-PHD2 domain of ZFP-1/AF10 is a discrete functional module essential for viability in elegans. Molecular and Cellular Biology. 2013 Mar;33(5):999-1015.
- Cecere, G., Zheng, G. X., Mansisidor, A. R., Klymko, K. E. and Grishok, A. Promoters recognized by forkhead proteins exist for individual 21U-RNAs. Molecular cell. 2012 Sep 14;47(5):734-45. doi: 10.1016/j.molcel.2012.06.021. (F1000 Prime Recommendation)
- Mansisidor, A.R.*, Cecere, G.*, Hoersch, S., Jensen, M.B., Kawli, T., Kennedy, L.M., Chavez, V., Tan, M.W., Lieb, J.D., and Grishok, A. A conserved PHD finger protein and endogenous RNAi modulate insulin signaling in Caenorhabditis elegans. PLoS genetics. 2011 Sep;7(9): e1002299. doi: 10.1371/journal.pgen.1002299. *co-first authors
- Cecere, G.*and Cogoni, C*. Quelling targets the rDNA locus and functions in rDNA copy number control. BMC microbiology. 2009 Feb 25; 9:44. doi: 10.1186/1471-2180-9-44. (Highly accessed articles) *co-corresponding authors
- Nolan, T.*, Cecere, G.*, Mancone, C., Alonzi, T., Tripodi, M., Catalanotto, C., and Cogoni, C. The RNA-dependent RNA polymerase essential for post-transcriptional gene silencing in Neurospora crassainteracts with replication protein A. Nucleic acids research. 2008 Feb;36(2):532-8. Epub 2007 Nov 29.(F1000 selection) *co-first authors