Link to Pubmed [PMID] – 21572003
J. Bacteriol. 2011 Jul;193(13):3186-96
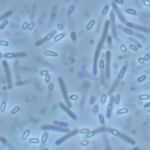
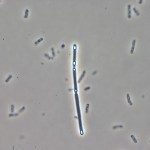
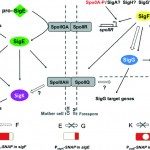
Toxin synthesis in Clostridium difficile increases as cells enter into stationary phase. We first compared the expression profiles of strain 630E during exponential growth and at the onset of stationary phase and showed that genes involved in sporulation, cellular division, and motility, as well as carbon and amino acid metabolism, were differentially expressed under these conditions. We inactivated the sigH gene, which encodes an alternative sigma factor involved in the transition to post-exponential phase in Bacillus subtilis. Then, we compared the expression profiles of strain 630E and the sigH mutant after 10 h of growth. About 60% of the genes that were differentially expressed between exponential and stationary phases, including genes involved in motility, sporulation, and metabolism, were regulated by SigH, which thus appears to be a key regulator of the transition phase in C. difficile. SigH positively controls several genes required for sporulation. Accordingly, sigH inactivation results in an asporogeneous phenotype. The spo0A and CD2492 genes, encoding the master regulator of sporulation and one of its associated kinases, and the spoIIA operon were transcribed from a SigH-dependent promoter. The expression of tcdA and tcdB, encoding the toxins, and of tcdR, encoding the sigma factor required for toxin production, increased in a sigH mutant. Finally, SigH regulates the expression of genes encoding surface-associated proteins, such as the Cwp66 adhesin, the S-layer precursor, and the flagellum components. Among the 286 genes positively regulated by SigH, about 40 transcriptional units presenting a SigH consensus in their promoter regions are good candidates for direct SigH targets.