Link to Pubmed [PMID] – 26890680
Nature Protocol 2016 Mar;11(3):525-41Epub 2016 Feb 18
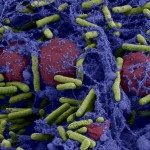
Venous access catheters used in clinics are prone to biofilm contamination, contributing to chronic and nosocomial infections. Although several animal models to study device-associated biofilms were previously described, only few detailed protocols are currently available. Here, we provide a protocol using totally implantable venous access port (TIVAP) implanted in rats. This model recapitulates all phenomena observed in the clinic and allows bacterial biofilm development and physiology to be studied. After TIVAP implantation and inoculation with luminescent pathogens, in vivo biofilm formation can be monitored in situ and biofilm biomass can be recovered from contaminated TIVAP and organs. We used this protocol to study host responses to biofilm infection, to evaluate preventive and curative anti-biofilm strategies, and to study fundamental biofilm properties. For this procedure, one should expect ~3h of hands-on time including the implantation in one rat followed by in situ luminescence monitoring and bacterial load estimation.