Link to Pubmed [PMID] – 30131361
MBio. 2018 Aug 21;9(4). pii: e01415-18. doi: 10.1128/mBio.01415-18.
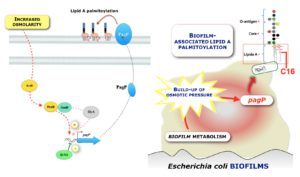
The development of dense bacterial communities called biofilms creates a highly heterogeneous environment, in which bacteria are subjected to a variety of physicochemical stresses. We investigated the mechanisms of a widespread and biofilm-associated chemical modification of the lipopolysaccharide (LPS), a major component of all Gram-negative bacterial outer membrane. This modification corresponds to the incorporation, mediated by the enzyme PagP, of a palmitate chain onto lipid A (palmitoylation) that reduces bacterial recognition by host immune responses. Using biochemical and genetic approaches, we demonstrate that a significant part of biofilm-associated lipid A palmitoylation is triggered upon induction of pagP transcription by the hyperosmolar biofilm environment. pagP induction is regulated by RcsB, the response regulator of the Rcs stress response pathway and is not observed in planktonic conditions. Our study provides new insights into how physiological adaptations to local biofilm microenvironment can contribute to decrease susceptibility to antimicrobial agents and host immune defenses.